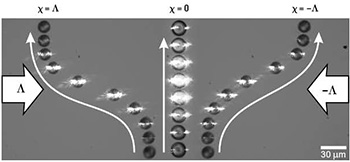
 Successive snapshots show the deviation of chiral and non-chiral nematic liquid crystal droplets, with respective chirality parameters χ = ±1 and χ = 0, flowing through two counter-propagating light beams with opposite helicity Λ and −Λ, with Λ = ±1.
Successive snapshots show the deviation of chiral and non-chiral nematic liquid crystal droplets, with respective chirality parameters χ = ±1 and χ = 0, flowing through two counter-propagating light beams with opposite helicity Λ and −Λ, with Λ = ±1.
A chiral system is one that lacks mirror symmetry—that is, a system with “left handed” and “right handed” variations (enantiomers) that cannot be superimposed by mere rotations and translations in space. Chirality is a fundamental property of material systems, in particular living ones, which tend to be characterized by homochirality. This can have important consequences. For example, drug development often requires pure chiral substances, yet drug synthesis usually results in equal amounts of left- and right-handed enantiomers (a racemic mixture), so that a final purification or separation step is required.
Chiral sorting also lies at the heart of the development of future technologies, such as those relying on carbon nanotubes, for which the possible chirality of their rolled structure helps to determine some of their electronic and optical properties. But the separation of enantiomers at the microscopic scale is a long-standing problem—going back more than 160 years, when Louis Pasteur used tweezers to resolve racemic acid by hand.1 This seminal experiment led to the advent of stereochemistry and, to date, enantioseparation of microscopic chiral entities remains a paradigmatic scientific and technological issue.
Established molecular chiral separation techniques rely mainly on the selective interaction between the chiral analyte of interest and an appropriate recognizing chiral agent.2 The requirement of a tailor-made chiral selector constitutes a serious drawback, implying ever-increasing synthesis efforts, but no competitive alternative yet exists, and much current effort focuses on finding alternatives to established chiral separation techniques. Indeed, replacing Pasteur’s handmade, macroscopic approach with a passive, contactless, microscopic technique would establish new standards in enantioseparation. To date, only a few experimental attempts—most of them relying on hydrodynamic forces associated with the chiral shape of the object to be sorted—have been reported, and these have been limited to above the micron scale.
In 2014, we proposed an approach to do chiral sorting using chiral light.3 More precisely, we have shown experimentally that material chirality can be passively sorted in a fluidic environment by chiral light, owing to optical forces that depend on the photon helicity, without a chiral morphology prerequisite.4 A proof-of-principle demonstration involved the sorting of optomechanically resonant chiral droplets made of a chiral nematic liquid crystal, though non-resonant droplets can be sorted as well (albeit less efficiently).3
These results bring a new twist to the state-of-the-art optofluidic toolbox, and could spur development of novel, passive integrated optofluidic sorters to deal with molecule-scale entities. As a first step in that direction, we have extended the concept to selective 3-D trapping of chiral particles.5
Researchers
Georgiy Tkachenko and Etienne Brasselet, Université de Bordeaux, Talence, France
References
1. L. Pasteur. Annales de Chimie Physique 24, 442 (1848).
2. T.J. Ward and B. A. Baker. Anal. Chem. 80, 4363 (2008).
3. G. Tkachenko and E. Brasselet. Nat. Commun. 5, 3577 (2014).
4. G. Tkachenko and E. Brasselet. Phys. Rev. Lett. 111, 033605 (2013).
5. G. Tkachenko and E. Brasselet. Nat. Commun. 5, 4491 (2014).
