 [Getty Images]
[Getty Images]
In May 2021, the United Nations (UN) General Council formally approved the designation of 2022 as the UN International Year of Glass (IYoG)—an opportunity to celebrate the material’s essential role in society. The IYoG celebration comes only seven years after another International Year familiar to OPN’s readers: the 2015 UN International Year of Light.
For the advanced fiber optic applications of the future, glass science and engineering are more important than ever.
Indeed, glass and light have had a remarkably intertwined history—from the glass artifacts of antiquity to lenses for microscopes and telescopes; from the windows in houses and buildings to the optical fibers that connect virtually everyone (and everything) on Earth. This feature looks at the role of glass in optical fiber in particular—and seeks to illuminate the many reasons to be excited about that pairing in the years ahead. For the advanced fiber optic applications of the future, glass science and engineering are more important than ever. [View timeline "A brief history of glass, light and optical fiber."]
“It’s just glass”
A common refrain in the laser and photonics community—at least anecdotally—is that glass “is just glass.” Glass often is considered simply a thing that one heats and pulls on to produce an optical fiber. Beyond a dash of some component to modify, for example, the refractive index, glass is otherwise a pedestrian commodity. Before dispelling this misconception, it is useful first to define what glass is and what it is not.
In its most basic definition, glass is a non-crystalline solid that exhibits a glass transition. Non-crystalline here means that the molecular structure lacks the long-range periodicity of a crystal. Glasses exhibit only a short-range order, on the scale of just a few molecular units. Compared with any meaningful wavelengths of light, glass structure is randomly arranged.
 It was long believed, based on thickening of cathedral stained glass toward the base, that glass could flow slowly at ambient temperatures. In fact, as E.D. Zanotto showed in a classic paper, the viscosity of glass is so high that that any such flow takes place on timescales that “exceed the limits of human history.” [Stained glass at St. Gatien Cathedral, Tours, France / Getty Images]
It was long believed, based on thickening of cathedral stained glass toward the base, that glass could flow slowly at ambient temperatures. In fact, as E.D. Zanotto showed in a classic paper, the viscosity of glass is so high that that any such flow takes place on timescales that “exceed the limits of human history.” [Stained glass at St. Gatien Cathedral, Tours, France / Getty Images]
Glass transition refers to a kinetic phenomenon where the volume (or other representative thermodynamic property) of a glass, as function of temperature, deviates from “liquid-like” behavior above a so-called glass-transition temperature, Tg, to “solid-like” behavior below it. In this definition, solid-like refers to the practical consideration that, while the liquid-like molecular structure of a glass may in theory permit it to relax over time, the viscosity is so astronomically high at room temperature that the time constant for measurable dimensional change is well beyond the age of the universe.
Cool a molten glass quickly, and its Tg value is higher and its density is lower than if cooled more slowly. This cooling-rate dependence on physical properties defines glass as a kinetic creature; it is also a fundamental enabler of all optical fiber. But glass does not stop here. Two other enabling qualities unique to glasses have significant consequences for the existence of optical fiber.
First, the structural order of a crystal demands a chemical order as well—meaning that crystals must possess a specific stoichiometry. For example, the very common solid-state laser crystal YAG is chemically Y3Al5O12. As such, nature mandates the entire crystal must possess a ratio of 3 yttrium atoms to every 5 aluminum atoms to every 12 oxygen atoms. Further, each individual building block, or unit cell, for YAG consists of 160 atoms, repeated ad nauseum, with the requisite ratios.
Glass, in contrast, isn’t chemically bound by rigid stoichiometric rules, because of its amorphous structure. The randomly arranged constituent molecular units in glasses can accommodate a very wide range of compositions. Glasses are, quite literally, nature’s melting pots.
That inherent compositional flexibility means a proportional flexibility in physical properties. Conventional optical fibers require a higher-refractive-index core surrounded by a lower-index cladding. Ideally, the core and cladding possess similar thermomechanical properties to minimize issues when co-drawn from the preform into the fiber. The flexibility in the physical properties of glass permits a staggering range of possible optical-fiber compositions and, therefore, properties and performance.
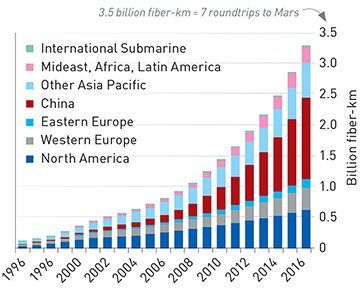 Cumulative total of cabled fiber installed worldwide. [Enlarge graph] [P. Schultz, from Optica webinar based on CRU data]
Cumulative total of cabled fiber installed worldwide. [Enlarge graph] [P. Schultz, from Optica webinar based on CRU data]
Second, the random molecular structure of glass also yields a viscosity that continuously changes with temperature above the glass transition. This is a phenomenon unique to glasses. Crystals are rigid solids below their melting point; glasses can viscously deform at lower temperatures—and do so in a very controllable way. Given this dependence, glasses do not, per se, have a melting point. It is this very effect that allows the drawing of an optical fiber from a preform or the tapering of a fiber to even smaller dimensions.
Optical fiber’s ubiquity thus rests, in a very real sense, on the unique properties of glass. But nothing in the above suggests that glass has to be made from silica, the material that constitutes the billions of kilometers of communications fiber made to date. Nor is it stated that glass must be an inorganic, nonmetallic material (that is, a ceramic). Indeed, there are metallic glasses, organic glasses, polymer glasses and other varieties; polymer optical fibers are important glass systems, too. The defining characteristics of glasses are structural and kinetic.
“If you can use silica, use silica”
That said, if you can use silica, use silica. From a glass science perspective, silica is chemically stable, very strong and very, very clear, making it ideal for applications ranging from ocean-spanning communication cables to fiber sensors operating under the most extreme down-hole conditions. From a glass engineering perspective, the development of chemical vapor deposition (CVD) techniques, which permitted the fabrication of preforms from which fiber could be drawn with essentially the strength and transparency predicted by theory for an ideal glass, is arguably amongst the greatest practical achievements of all time.
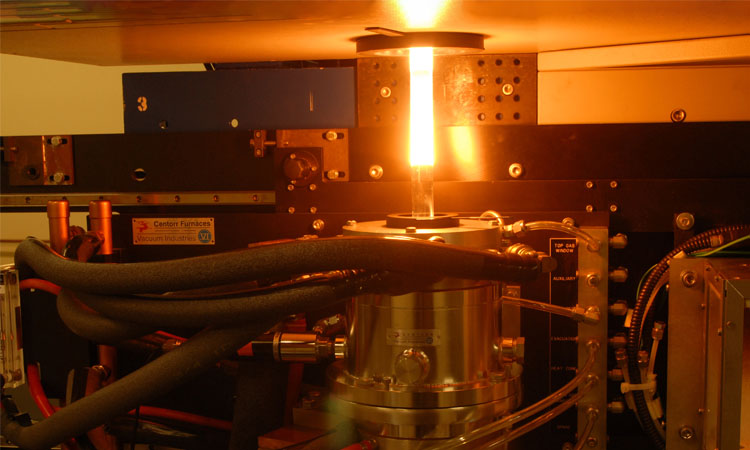 Optical-fiber draw tower, Clemson University, SC, USA. The combination of glass’s material properties and the chemical vapor deposition method for creating preforms enabled the drawing of hair-thin, ultra-high-quality optical fibers—an achievement of enormous practical importance. [Clemson University]
Optical-fiber draw tower, Clemson University, SC, USA. The combination of glass’s material properties and the chemical vapor deposition method for creating preforms enabled the drawing of hair-thin, ultra-high-quality optical fibers—an achievement of enormous practical importance. [Clemson University]
In the past few years, several OPN feature articles have recounted how silica fiber’s properties for optical communication were discovered and perfected (see OPN, March 2019 and March 2020). Here, in sync with IYoG, a different story warrants telling—how the seeming simplicity of silica can beget an underlying sophistication, and how, in practice, glass science and engineering seemingly can be at odds.
For example, if anything can form a glass, why is it that less than 10% alumina (Al2O3) can be added into a CVD silica preform, but over 50% can be realized in silica fiber made via other methods? The answer is as commercially important as it is scientifically interesting.
As already noted, glass is a kinetic creature, cooled to a rigid form without crystallizing, as thermodynamics demands. This also means that glass is fundamentally unstable. However, as with (crystalline) diamond, which is thermodynamically unstable under ambient conditions, the metastability timescales of glass are more than sufficient for us never to need worry that the glass will devitrify or the diamond turn to coal.
But, in kinetics, time and temperature are nature’s primary tools. If you can’t wait for geologic timescales, then heat things up. Put another way, silica at room temperature is metastable for a practically infinite amount of time—but heat it to 2000 °C and you can draw optical fiber at speeds faster than the eye can see. This is a direct consequence of glass’s (viscous) liquid-like behavior above the glass transition, enabled by its amorphous structure.
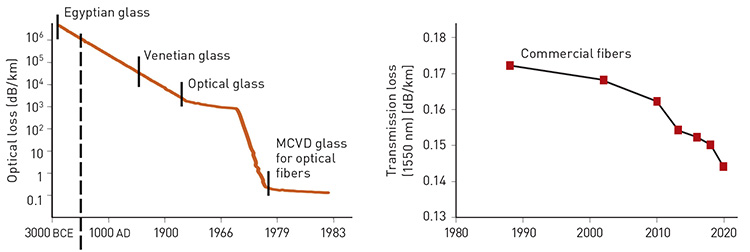 The evolution of optical loss over time. [V. Romano et al., in G.D. Peng, ed., Handbook of Optical Fibers (Springer, Singapore, 2018), reprinted by permission of Springer; Adapted from T. Hasegawa et al., SEI Tech. Rev. 86, 18 (2018)]
The evolution of optical loss over time. [V. Romano et al., in G.D. Peng, ed., Handbook of Optical Fibers (Springer, Singapore, 2018), reprinted by permission of Springer; Adapted from T. Hasegawa et al., SEI Tech. Rev. 86, 18 (2018)]
While this fundamentally enables optical-fiber fabrication, it has detrimental effects as well. When one mixes two liquids together, sometimes they mix well (coffee and creamer) and sometimes they do not (oil and water). The same is true with glass constituents. SiO2 and GeO2, an important index-raising and nonlinear-optical constituent, are completely miscible (mutually mixable), so in principle one could make a glass from any combination of these two. Not so with Al2O3; add Al2O3 into SiO2 in one of several immiscible compositional ranges, and you risk having the phases separate like oil and water.
So, in answer to the question of why CVD permits alumina concentrations in silica of less than 10%, whereas other processes such as the molten-core method permit more than 50% in silica, the reason is the kinetics of glass formation. The combination of time (for example, transverse speed and rotation rate) and temperature of the CVD lathe multiple deposition, consolidation and collapse stages, which takes the glass on numerous excursions through regions of metastability and instability, leads to these compositional restrictions. The “ecstasy” of CVD fiber performance begets the “agony” of its glass-forming limits (see “120 Years of Optical Glass Science,” OPN, May 2014). The take-home message is that Mother Nature always wins.
Through the looking glass
Since one can’t beat Mother Nature, then one might as well join her. This section discusses a series of future opportunities for optical fiber uniquely enabled by glass science and engineering.
It is worth a reminder that the performance of an optical fiber is determined both by its design (for example, single mode, multimode, graded index, photonic crystal, anti-resonant and so forth) and the materials from which it is made. Whereas the former relates to the boundary conditions on Maxwell’s equations, the latter relates to the constitutive equations that represent the matter part of light–matter interactions—where Maxwell meets material.
From the specific perspective of glass science and engineering, there are several noteworthy trends relevant to future advancements in optical fibers.
From the specific perspective of glass science and engineering, there are several noteworthy trends relevant to future advancements in optical fibers.
One in which glass can play an outsized role, given opportunity and greater awareness, is in response to trends in optical fibers operating at very high intensities. Applications here range from high-energy fiber lasers (such as in multi-kW-class continuous-wave directed-energy systems), to high-peak-power pulsed lasers (such as those used for lidar), to high-intensity-per-unit-bandwidth lasers (such as in highly multiplexed communication links). While different in many ways, these applications share a common need to manage two things: parasitic optical nonlinearities and heat.
Managing nonlinearities
Regarding the nonlinearities, an especially useful glass-based mitigation approach is to take advantage of glass’s compositional flexibility—in a fascinating way. It is well known, for example, which added compounds raise or lower silica’s refractive index relative to the SiO2 base value. When it comes to other properties, however, some constituents possess negative-valued coefficients.
The canonical example is photoelasticity. Pure silica possesses a positive (transverse) photoelastic coefficient, but, interestingly, alumina’s is negative-valued. Mix the two and the effective photoelasticity can be significantly reduced—and even, at some compositions, negated entirely. This same balancing of positive- and negative-valued constituent effects can be employed for the thermo-optic coefficient as well.
This becomes important for optical nonlinearities in that the (transverse) photoelastic effect directly relates to Brillouin scattering, whereas the thermo-optic effect directly drives transverse mode instability (TMI). Stimulated Brillouin scattering (SBS) and TMI are the two most consequential nonlinearities that plague power-scaling in many high-power fiber-laser applications, and their influence can be mitigated through glass science.
Managing heat
Glasses also offer unique opportunities regarding heat generation and thermal management. While lasing is naturally exothermic (heat generating), both the magnitude of the heat generated and the resultant effect that heat has on a fiber’s optical properties are directly tailorable through glass composition.
In fiber amplifiers and lasers, heat arises from several sources associated with the active dopant, including nonradiative relaxations and the quantum defect. These two sources alone can account for 10% or more of the overall power budget. Fluorine doping, for instance, is known to reduce the quantum defect, and doping with heavier atoms of the same valency (for example, La2O3 for Al2O3) or column of the periodic table can directly modify the host phonon energy, which increases the likelihood for radiative emission and reduced heat generation.
Further, as noted, glass composition can have a major influence on the thermo-optic coefficient. If suitably reduced, as by doping the SiO2 with P2O5 or B2O3, the change in the fiber’s optical properties as it heats can be made much smaller. Here, too, glass affords even more unique and advantageous opportunities: If the core has a much lower thermo-optic coefficient relative to the cladding, a large-mode-area (LMA) fiber may be few-moded at low power and, as its temperature rises with output power scaling, eventually become single moded.
Such thermal self-single-moding is entirely passive and originates from the differential properties of the core and cladding glasses. Most generally, optical nonlinearities and heat generation in active fibers originate from many of the same light–matter interaction processes and, so, can be mitigated in the same way.
Looking beyond silica
With apologies to “If you can use silica, use silica,” what if you can’t? A growing list of applications require non-silica-based fibers—for reasons related to spectral range (for example, a need for light generation or transmission at wavelengths for which silica is ill-suited) or nonlinearities (when wave-mixing or nonlinear-scattering effects are employed for specific purposes). Those who attended OFC in the late 1990s and early 2000s will remember the great efforts that went into fluoride glass fibers for optical amplifiers operating at 1.3 mm and for potential ultra-low-loss fibers.
Those promises were never realized due to the specifics of fluoride glass science and engineering relative to those of silica. The material properties of fluorides that give rise to their extended infrared (IR) transparency and theoretically low attenuation values, also make them relatively weak and ill-adapted to the high-volume manufacturing methods required for scale.
However, while fluorides were not up to the challenge for long-haul telecom, they definitely are for certain specialty uses. The weaker ionic bonding between relatively heavy constituents, compared with SiO2, enables broad IR transparency and low nonradiative-relaxation rates for active dopants. These properties make fluorides a nearly ideal glass host for a new generation of IR fiber lasers and supercontinuum sources. Chalcogenide glasses likewise are finding growing appeal and utilization for more highly nonlinear and mid-to-long-wavelength IR fiber-based light sources and imaging arrays. In the case of these specialty glasses, the trick was finding the right applications at the right time.
If the absence of silica makes one uncomfortable, opportunities exist with silica-clad, semiconductor-core optical fibers. As already noted, a unique feature of glass is that it can be deformed above its glass-transition temperature and that its viscosity uniformly changes with temperature. Crystals such as semiconductor materials, in contrast, do not soften—they are solid until they reach their melting temperature, at which point they become fluid. Thus they cannot benefit from the scalability of fiber draw. In multimaterial fibers, the role of silica glass is to permit what otherwise is not permissible: the drawing of a crystal.
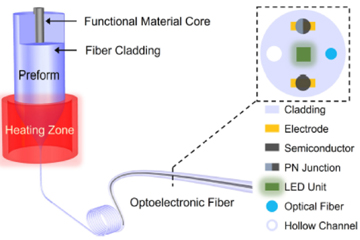 Fiber optics meets silicon photonics: Modern drawing techniques that allow silicon, germanium and other semiconductor materials to form the core of optical fibers raise the prospect of semiconductor devices embedded directly in fiber. [Enlarge graph] [J. Zhang et al., J. Lightwave Technol. 39, 3836 (2021)]
Fiber optics meets silicon photonics: Modern drawing techniques that allow silicon, germanium and other semiconductor materials to form the core of optical fibers raise the prospect of semiconductor devices embedded directly in fiber. [Enlarge graph] [J. Zhang et al., J. Lightwave Technol. 39, 3836 (2021)]
This is most practically done by sleeving the semiconductor inside a glass tube, sealing the tube at one end, and heating to a temperature at which the crystalline semiconductor melts and the glass softens and draws into fiber. Upon cooling of the fiber, the molten semiconductor core solidifies into a crystalline state. Such drawn semiconductor optical fibers have been made from a growing range of materials, including silicon, germanium, indium antimonide, gallium antimonide and silicon–germanium alloys, to name but a few.
Such fibers marry the vast opportunities of silicon photonics with fiber optics. They yield very strong nonlinearities and infrared transparencies inherent in semiconductors, in a glass package that is compatible with conventional silica fibers (see “In-Fiber Silicon Photonics,” OPN, March 2019).
How low (loss) can you go?
Returning to silica, two opportunities are particularly exciting. Despite it now being more than 50 years after the first low-loss fibers were reported, with billions of kilometers manufactured and laid since then, silica still has some secrets to yield.
Virtually every OFC conference features reports of hard-fought battles to eke out even a fraction of a dB/km lower loss each year, because this significantly impacts system reach and cost over long-haul distances. The approaches for hammering down loss rely on silica glass science and engineering—and, specifically, tailoring Rayleigh scattering. The glass-science aspect involves, among other approaches, going to pure silica cores and a fluorine-doped cladding. The glass-engineering aspect involves thermal treatments to relax the glass structure and reduce the Tg value, to make the glass slightly less “liquid-like.” A fascinating question is “How low can you go?” The beauty of glass is that there is not necessarily a definitive answer.
Despite it now being more than 50 years after the first low-loss fibers were reported, with billions of kilometers manufactured and laid since then, silica still has some secrets to yield.
A second opportunity is the remarkable advances in hollow-core, anti-resonant fibers that guide light in air confined by a narrow web of glass tubes (see “Is Nothing Better than Something,” OPN, March 2021). Such fibers are of interest for low-latency communications and have recently accomplished the nearly unthinkable—exhibiting lower losses than silica, at least in selected spectral ranges.
Here again, though, Mother Nature says hello. The liquid-like nature of the glass leads to capillary instabilities during the draw. While these perturbations are small at the higher draw tensions employed, they are omnipresent and promote scattering, which imposes an lower limit on attenuation. Regardless of the light primarily propagating in the hollow core, it is still the glass that enables the structure and defines its performance limits. While such instabilities are thermodynamic, as with many other aspects of glass, they can be controlled through kinetics. There is no doubt such glass-enabled optical fibers are here to stay and will only continue to improve.
The practical applications of glass are many millennia old, yet glasses still remain among the most enabling of present and future technological materials. Photonic technologies generally, and fiber optics ones particularly, are foundationally dependent on glass science and engineering. And despite 50 years of low-loss fibers and the billions of kilometers manufactured, glass arguably is more exciting now than it has ever been—precisely because glass isn’t “just glass.” Even in those cases where the glass is a minor component of the fiber, its effect is still felt. For many of the most pressing challenges facing fiber optics, glass will continue to offer solutions. Befriend a glass scientist, and let nature do the work for you.
Optica Fellow John Ballato is with the Department of Materials Science and Engineering, Clemson University, Clemson, SC, USA.
References and resources: optica-opn.org/link/lk-glass
