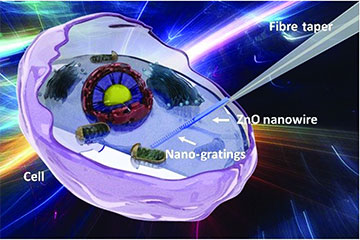
Illustration of a zinc oxide nanowire with etched gratings mounted on a fiber taper that can image inside live cells without chemical labels and without damaging the cell. [Image: Li et al., Adv. Photon. 4, 016001 (2022), doi: 10.1117/1.AP.4.1.016001; CC BY 4.0] [Enlarge image]
A research team from Nanjing University, China, created an intracellular fiber sensor that can measure changes in refractive index (RI) within a cell as it dies—without chemical labels and with minimal damage to the cell (Adv. Photon., doi: 10.1117/1.AP.4.1.016001). The device consists of a biocompatible zinc oxide (ZnO) nanowire attached to a flexible fiber-optic taper. Etched gratings on the nanowire act as a waveguide to passively transmit light to and from the inside of the cell, eliminating the need for potentially harmful fluorescent markers.
In demonstrations, the optical nanoprobe sensed an increase in RI within live cells during programmed cell death, or apoptosis, indicating a loss of cell volume and an increase in molecular density as cells mature and die. Data on the physiological changes that occur during apoptosis can improve our understanding of cellular function and disease, according to the team.
Design and experimental setup
The researchers chose ZnO for the nanowire because it is biocompatible, has low transmission loss, and has high RI—qualities needed for strong light-signal confinement in fluid environments like the inside of a cell. They transformed the 800-nm diameter ZnO nanowire into a waveguide by etching 660-nm gratings onto its surface. The nanowire was then attached to a fiber-optic taper. With optical nanogratings in the visible spectrum of light, the nanowire was designed to simultaneously receive and send optical signals via the taper while inside a live cell.
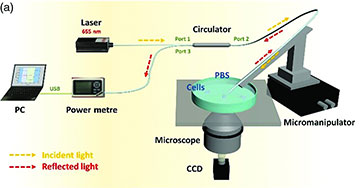
Experimental setup used to measure the intracellular refractive index (RI) of live human breast cancer cells. A low-power laser at 655 nm is injected through a fiber-optic taper into a zinc oxide nanowire etched with gratings. Output light reflected at the gratings is transmitted to an optical power meter and computer to record the reflection power and RI. [Image: Li et al., Adv. Photon. 4, 016001 (2022), doi: 10.1117/1.AP.4.1.016001; CC BY 4.0] [Enlarge image]
The Nanjing team’s experimental setup for measuring intracellular RI in live human breast cancer cells included a micromanipulator, microscope and CCD camera to orient the ZnO nanowire into the cell. Low-power laser light (655 nm) traveled through the fiber-optic taper and into the cell via the ZnO nanogratings. Light reflected at the gratings was transmitted back through the taper and into an optical power meter and computer that recorded the reflection power and converted it to RI.
Measuring RI in situ
To demonstrate the device’s ability to measure changes in RI that occur during cell death, the researchers inserted the ZnO nanowire into three live human breast cancer cells. These three cells and several other non-probed cells were treated with hydrogen peroxide to trigger apoptosis—a genetically programmed process that occurs when cells mature and die.
The researchers took RI measurements from the three probed live cells at four different time points: immediately on treating the cells with hydrogen peroxide, and 10, 30 and 40 minutes after the treatment. At the 40-minute mark, they used fluorescence microscopy on the three probed and the un-probed cells to confirm cell death and to see if using the nanowire has an effect on how fast the cells died.
RI measurements from the three probed cells gradually increased, indicating a decrease in cell volume and an increase in molecular density during apoptosis. The fluorescence intensity for all cells at 40 minutes was consistent, indicating that the nanowire did not influence apoptosis.
Based on these initial findings, the researchers are hopeful that their device will provide a new platform for investigating biochemical processes that occur within live cells.