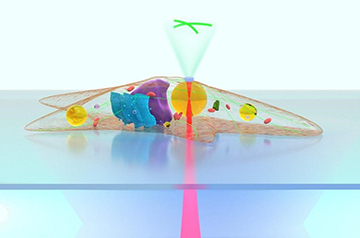
An artist’s view of a lipid droplet inside a cell focusing and collecting light to form a magnified fluorescence image. [Image: X. Chen et al.]
Researchers from China and Italy have developed and demonstrated a method for using naturally occuring lipid droplets inside adipose cells as microlenses, to view and monitor intracellular structures and extracellular fluorescence signaling (Light Sci. Appl., doi: 10.1038/s41377-021-00687-3).
Compared with traditional fluorescence microscopy, the team’s demonstrations showed that imaging with the biophotonic microlens increased image resolution and signal strength while requiring substantially less light, which can damage and kill live samples. The researchers hope to see their technique used in real-time detection of cancer, viral infection and other diseases.
Fluorescence imaging, au naturel
Fluorescence microscopy is commonly used to monitor intracellular variation and extracellular signals in real time. However, fluorescence signals from subcellular structures are generally weak and difficult to detect. Pumping up the excitation light intensity can improve fluorescence signal strength—but it also increases the chance of phototoxicity and photobleaching.
Researchers from the Institute of Nanophotonics, Jinan University, China, and the Institute of Applied Sciences and Intelligent Systems, Italy, decided to look for a more natural approach to improving fluorescence imaging of subcellular structures. They zeroed in on using endogenous lipid droplets in living cells as microlenses to enhance imaging. Because the lipid spheres are naturally occurring, they’re fully biocompatible
Prior to demonstration experiments, the researchers characterized lipid droplets inside adult adipose cells (more commonly called fat cells). The droplets were smooth and spherical, with diameters ranging from 0.5 to 40 µm and a refractive index around 1.52. The latter number is higher than the index for cyto- and periplasm; that made the lipid droplets a potentially good fit for intracellular fluorescence imaging and for detecting extracellular fluorescence signals.
To drive the lipid microlenses through the cytoplasm to image different structures inside and outside the cell, the researchers found that optical tweezers of 1064 nm were ideal, nudging along the droplets while causing minimal damage to the cell. The experimental setup for the microlens demonstrations consisted of a scanning optical tweezing system with an inverted optical microscope for detecting bright-field and fluorescence signals.
Looking inside and outside
For intracellular bright-field-imaging demonstrations, the structures to be viewed were positioned below the lipid droplet in the cytoplasm, allowing the droplet to lightly press against the structure. In these preliminary contact-mode tests, the researchers discovered that the droplets could resolve structural features 100 nm in size, with a stronger signal and using significantly less excitation power compared with traditional fluorescence microscopy.
The team was able to image and track, in real time, fluorescently labeled actin filaments in the cytoskeleton, as well as lysosomes and adenoviruses migrating through the cytoplasm, for 10 minutes.
To demonstrate extracellular fluorescence signal detection using the lipid lenses, the researchers exploited the lipid droplet’s long focal length and high refractive index. In this non-contact mode, they could use the lipid microlens to identify fluorescence signals outside the cell wall and into the surrounding microenvironment and tissues. In characterization studies with specific wavelengths of light, they found focal length increased as the lipid droplet diameter increased. For example, an excitation light with a wavelength of λ = 473 nm going through a lipid microlens more than 2 µm in diameter had a focal length greater than 10λ.
Toward better in vivo signal detection
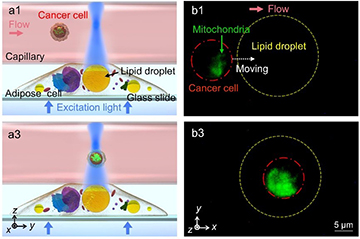
Illustration and fluorescence images from a demonstration of an intracellular lipid microlens detecting extracellular signals from a fluorescently labeled cancer cell flowing through a capillary. [Image: X. Chen et al.] [Enlarge image]
Finally, in a demo of enhanced fluorescence signal detection, the researchers used a glass capillary tube as a stand-in for a blood vessel, and coated it with adipose cells containing the lipid microlenses. They centered the microscope objective at the middle of a specific lipid microlens to concentrate the excitation light and the middle of the glass capillary
Next, the team passed a solution containing blood cells and fluorescently labeled cancer cells through the capillary and transmitted a beam of excitation light into the lipid microlens. The tiny lipid lens concentrated the excitation light, resulting in an enhanced, easy-to-detect fluorescence signal when the beam collided with a labeled cancer cell as it flowed through the tube.
In their recently published study, the researchers say they hope their microlens method can someday be used to develop endogenous photonic devices as well as “biocompatible optics tools for biosensing, endoscopic imaging, and single-cell diagnosis.”