A radium atom and polyatomic molecule are held between the electrodes of an ion trap. A team at the University of California, Santa Barbara, USA, will use the radium molecule to search for time-symmetry violation. [Image: Max Ladabaum]
Experimental optical clocks—based on atomic transitions that emit visible light instead of microwaves—employ atoms of metallic elements such as ytterbium, strontium and calcium. Now, researchers at a US university have built and operated an optical clock containing a single trapped radium-226 ion (Phys. Rev. Lett., doi: 10.1103/PhysRevLett.128.033202).
Besides being the first timekeeper based on the radioactive metal radium, the research team used its new optical clock to make the first measurement of the ratio of two quantities related to the angular momentum of the electric quadrupole transition within the radium ion. With further refinements, the clock could make other precise measurements related to fundamental quantum physics.
Single ion in a trap
The physicists at the University of California, Santa Barbara (UCSB), USA, began by ablating a single radium-226 ion from a sample of radium chloride and trapping the ion. The team then focused a linearly polarized 728-nm laser beam at the trapped atom, with the beam at a 45-degree angle to the trap’s magnetic field. The beam’s wavelength matched that of ion’s 7s2S1/2 → 6d2D5/2 quadrupole clock transition.
The radium ion scattered 468-nm photons, which were then collected by a photomultiplier tube. By self-referencing the ion’s Zeeman transitions, the researchers found that the clock had a frequency instability of 1.1 × 10-13 / τ1/2, where τ represents the averaging time in seconds. The device’s total systematic uncertainty was in the ballpark of 9 × 10-16.
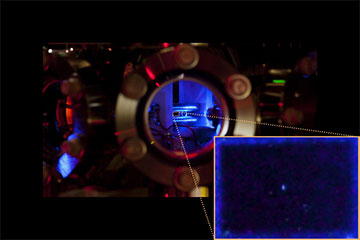
UCSB doctoral student Mingyu Fan used a long exposure to capture an image of this single radium ion caught in the team’s ion trap. [Image: Mingyu Fan]
Significance and prospects
According to Craig A. Holliman, a UCSB graduate student in the laboratory of a physicist Andrew M. Jayich, the radium-226 ion is a good choice for an optical clock for three reasons. First, it can “realize high accuracy on its narrow-linewidth electric quadrupole transition,” Holliman says. Second, its transition wavelengths are compatible with existing integrated-photonics technology. Third, radium-226 “has a high sensitivity to potential time variation of fundamental constants,” he adds.
Although the radium ion had been previously proposed for a single-ion optical clock, it had never been built until now, and Holliman suspects that was due to the risks of handling radioactive materials. “Also, before it could have been used in a clock, a group would have had to perform the first laser cooling of the ion and measure several fundamental atomic properties, and that is a big investment (lasers, vacuum equipment, etc) for something not guaranteed to work,” he adds.
Holliman predicts the radium-ion optical clock “will be able to achieve comparable or potentially even better performance than other alkaline-earth-ion optical clocks as the radium ion has nice properties for achieving a low total systematic uncertainty.”
Jayich and his lab colleagues first demonstrated laser cooling of radium ions in 2019. Now the team has set its sights on other goals: building a transportable optical clock and a long-lived radium source. The researchers also want to use the technology to probe the time variation of the fine-structure constant, one of the fundamental numbers in quantum physics.
