A research team in India has prototyped a directly solar-rechargeable battery using a novel semiconductor heterostructure. In the experiment shown here, the prototype is recharged under green light from a low-power LED. [Image: A. Kumar and T.N. Narayanan] [Enlarge image]
As concerns about climate change and fossil fuel use have increased, engineers have sought ever more environmentally sustainable ways to recharge those now-ubiquitous energy-storage devices, lithium-ion batteries. But the standard approach—wiring up separate banks of external photovoltaic cells to pump charge into the batteries—has drawbacks in weight, size and energy conversion loss. And more direct approaches, involving photosensitive elements that are integrated into the battery architecture itself, have fallen short in one way or another.
A team at the Tata Institute of Fundamental Research–Hyderabad, India, has now demonstrated a directly photo-rechargeable battery that the researchers believe could overcome the problems of some earlier approaches (Small, doi: 10.1002/smll.202105029). The design is based on a clever heterostructure of 2D semiconductor materials that functions as a battery electrode. The heterostructure electrode’s broad surface area maximizes solar absorption, while careful band-gap engineering of the two semiconductor components manages the flow of solar-generated photoelectrons into the battery charging circuit.
At present, the battery is a proof-of-concept only, and not quite ready for integration into production-ready Li-ion power packs. But the researchers believe that the use of heterostructures such as the one they’ve demonstrated could suggest architectures for a new generation of stable, directly photo-rechargeable batteries—without the need for external solar cells.
Recharging by the sun
The quest for a directly rechargeable “solar battery” actually goes back many decades. In a 1976 paper in Nature, for example, Gary Hodes and colleagues at the Weizmann Institute of Science, Israel, proposed a three-electrode solar cell in which one of the electrodes handled photoconversion to electricity and the other two electrodes managed the actual energy storage and retrieval.
Since then, other researchers have proposed alternative designs, many of them incorporating photosensitive dyes, perovskite crystals or organic materials into battery electrodes, to generate photocurrents that could be used in recharging the battery cell. But these approaches have tended to limit the available surface area or wavelength range that can be used in photo-recharging, leading to low overall photoconversion efficiencies. And undesirable chemical reactions between the photo-responsive materials and the other battery components have cut operating lifetimes short.
Heterostructure solution
To get around these problems, the Tata Institute team behind the new work, led by Tharangattu N. Narayanan, looked at another approach: a semiconductor heterostructure. By leveraging the 2D configuration of thin, photosensitive materials such as molybdenum disulfide (MoS2), the team reasoned, one might create a very thin, compact photo-recharging module for a Li-ion battery. And such materials had already been shown to have a high stable energy capacity for many cycles of operation in conventional battery designs, implying a long operating lifetime.
Critically, making such a system work in a photo-rechargeable battery requires designing a heterostructure that can separate photo-excited electrons from positively charged “holes” left behind in the semiconductor. Otherwise, the electrons would simply re-combine with the holes, sabotaging the photo-recharging function.
To achieve that separation, Narayanan’s team hit upon a heterostructure of nanorods combining MoS2 and a molybdenum oxide semiconductor, MoOx. In such a “type II” heterostructure, the semiconductor band gaps of the two materials are staggered in such a way that the photoexcited electrons are sucked into the low-energy conduction band of one semiconductor—in this case, the MoOx—and are thus made available to the battery’s charging circuit. The holes, meanwhile, are left behind in the higher-energy valance band of the MoS2.
Home-built prototype
The team set about creating a prototype by first using chemical-vapor deposition to synthesize the MoS2/MoOx nanorod heterostructures. Next, the researchers spread a solution of the heterostructure material, a small amount of carbon black, and a polymer binder onto a transparent indium-tin-oxide (ITO) glass plate. They used Raman spectroscopy, X-ray diffraction and other techniques to characterize the chemical and crystal structure of the material, and confirmed its photocurrent under illumination with green, red and white light.
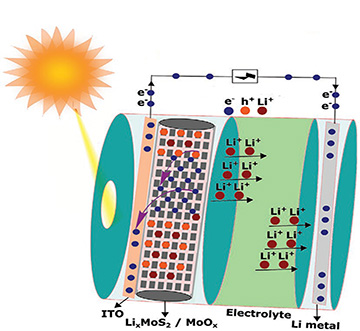
In the solar battery prototype from the Tata Institute team, sunlight passes through a transparent layer of indium-tin-oxide (ITO) and into a zone of MoS2/MoSx heterostructure nanorods. The photo-excited electrons are removed into the ITO layer and pass into the battery’s charging circuit. Positively charged holes remaining in the heterostructure MoS2’s valance band push positively charged lithium ions back toward a lithium anode, where they recombine with the electrons to form metallic lithium, re-charging the battery. [Image: A. Kumar and T.N. Narayanan] [Enlarge image]
With those tests done, the team next used the heterostructure plate as the cathode in a home-built model Li-ion battery, with a disk of metallic lithium as the anode. The model battery, with an open-circuit voltage of 3.1 V, was discharged in a dark room, which caused lithium ions to migrate from the anode to the cathode.
When the battery was then illuminated under simulated solar light, the photo-excited electrons were moved out of the MoS2 component of the heterostructure cathode and into the conduction band of MoOx component, where they flowed into the battery charging circuit. The positively charged holes remaining in MoS2 valence band in the cathode, meanwhile, pushed the positively charged lithium ions back toward the lithium anode. There, they combined with the liberated electrons to form lithium metal on the cathode, fully recharging the battery in the space of a few hours.
Toward new designs
While the proof-of-concept helped establish that semiconductor heterostructures might have a role in creating directly sun-rechargeable batteries in the future, there appears to be a quite a ways to go in making the system robust enough for commercial devices. Narayanan’s lab is now working toward a better understanding of the mechanisms at play, and how they might be tweaked to improve performance. Even so, the researchers conclude that the work thus far “opens up the potential in developing semiconductor heterostructures based novel photo-electrode designs in solar batteries.”