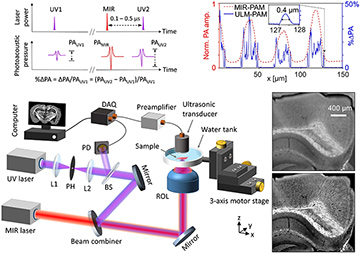
 Top left: Principle of ULM–PAM. Bottom left: Schematic of the experimental setup. BS, beam splitter; DAQ, data acquisition unit; L1, L2, lenses; PH, pinhole; ROL, reflective objective lens. Top right: Amplitude profiles of conventional MIR–PAM (red) and ULM–PAM (blue) images across a pattern of vertically aligned carbon nanotubes. Bottom right: MIR–PAM (upper image) and ULM–PAM (lower image) image of myelin in a mouse brain slice with a 300-μm thickness. [Adapted from reference 5.] [Enlarge figure]
Top left: Principle of ULM–PAM. Bottom left: Schematic of the experimental setup. BS, beam splitter; DAQ, data acquisition unit; L1, L2, lenses; PH, pinhole; ROL, reflective objective lens. Top right: Amplitude profiles of conventional MIR–PAM (red) and ULM–PAM (blue) images across a pattern of vertically aligned carbon nanotubes. Bottom right: MIR–PAM (upper image) and ULM–PAM (lower image) image of myelin in a mouse brain slice with a 300-μm thickness. [Adapted from reference 5.] [Enlarge figure]
Label-free mid-infrared (MIR) microscopy provides rich chemical and structural information on biological tissues. While traditional histopathology requires time-consuming tissue section processing and staining, MIR microscopy can obtain histopathologic information without staining. However, strong MIR absorption of water in fresh biological samples results in high background and low contrast, which limits conventional MIR techniques to imaging only dried and thin-sliced tissue specimens.1 Moreover, at long MIR wavelengths, optical diffraction severely limits lateral resolution, preventing the technique from resolving subcellular information. This year, we developed ultraviolet-localized MIR photoacoustic microscopy (ULM–PAM), which can achieve high-resolution and water background–free MIR imaging of fresh biological samples.2
Several techniques have previously attempted to address MIR microscopy’s limitations and thereby improve its performance. One recently developed approach, photothermal MIR imaging, employs a continuous-wave visible or near-IR laser beam to detect the refractive-index change due to the MIR absorption, thereby improving the resolution and reducing the water background.3 Because it is based on detection of scattered light, however, it works only for translucent samples or sample surfaces.
Another recently introduced method, MIR photoacoustic microscopy (MIR–PAM), can image totally opaque or highly light-scattering tissues with MIR absorption contrast,4 but it does not improve the spatial resolution or reduce the water background.
In the technique we developed, ULM–PAM,2 a pulsed MIR laser thermally excites the tissue specimen at the focal spot, and a pulsed ultraviolet (UV) laser photoacoustically detects the transient temperature rise owing to the Grüneisen relaxation effect,5 thereby reporting the MIR absorption by the sample. The UV laser’s wavelength—an order of magnitude shorter than that of a mid-IR laser—determines the imaging resolution. In addition, in the deep UV region (above 200 nm), most important organic molecules in biological tissues have strong absorption, while water is transparent. Therefore, ULM–PAM can achieve high resolution (around 260 nm) and water background–free MIR imaging of fresh biological samples—capabilities we demonstrated in cell cultures and thick biological tissues.2
By overcoming the limitations in MIR microscopy, ULM–PAM can benefit diagnosis of fresh biological samples. We believe that our work will promote the development of label-free histopathology in biomedical sciences.
Researchers
Junhui Shi and Lihong V. Wang, California Institute of Technology, Pasadena, Calif., USA
References
1. D.C. Fernandez et al. Nat. Biotechnol. 23, 469 (2005).
2. J. Shi et al. Nat. Photon. 13, 609 (2019).
3. D. Zhang et al. Sci. Adv. 2, e1600521 (2016).
4. J.Y. Sim et al. Sci. Rep. 8, 1059 (2018).
5. L. Wang et al. Phys. Rev. Lett. 113, 174301 (2014).
