
The July/August 2023 issue of Optics & Photonics News featured the magazine’s biennial feature spotlighting 10 Entrepreneurs to Watch. Here, we offer an interview with one of those entrepreneurs, Geethanjali Radhakrishnan, the founder and CEO of India-based Adiuvo Diagnostics. Adiuvo, a “techno-social enterprise,” aims to improve wound care in low-resource settings with its Illuminate device, which leverages the natural autofluorescence of bacteria and fungi for label-free multispectral imaging.
Can you start by telling me what the company is doing and where you’re looking to take it?
Geethanjali Radhakrishnan: I’m a bioengineer by background. And in India, we have different terrains, different kinds of ecosystems, so access to health care in the last-mile setting is always a challenge. So I really wanted to focus on building something that’s completely noninvasive and label free, and that can also give access to high-end quality diagnostics at the point of care. And what better way to do it than using optics and electronics combined? Because that’s the only way you can achieve a noninvasive way of detection.
“Adiuvo” means to help or to aid people. So that’s the core vision. And we want to build label-free solutions, which are completely reagent free, that can be used at the point of care and are specifically customized for low-resource settings. This solution is bound to work anywhere, even in a developed country. So that’s the whole idea.
Your company is described as a “techno-social enterprise.” Can you talk about what that means to you?
We wanted to, of course, create a social impact from whatever technology that we developed. But it should also be sustainable in the sense that we are a for-profit organization. We want to build a solution where we can measure the impact of each of the products that we develop, and figure out how best to reach somebody with a poor background. So that’s why we are funded by social-impact investors. And we use deep tech, so that’s why we are called a techno-social enterprise.
Is there anything you’ve done to track or encourage uptake of a new device like this in low-resource settings?
We were funded by Menterra Venture Fund and Lesing Artha limited, a Switzerland-based impact fund. The mandate behind them, which aligned well with our vision, is that at least a third of the revenue that we generate has to come from screening patients from a resource-poor setting.

So it’s actually in our mandate to have effective measurement and evaluation systems in place. When we sell these products in these low-resource setting areas, we have a good business model that works for them because they cannot afford high-end technologies or high-end screening costs.
Can you describe the core technology and what distinguishes Adiuvo’s approach from others in this market?
We are using multispectral imaging in a handheld way. What is very unique about our multispectral imaging is we are engaging the autofluorescence that comes naturally from bacteria.
You have heard about acute, chronic and traumatic wounds. These cover anything from burn victims to diabetic foot ulcer patients to post-surgery monitoring. Bacteria and fungi are everywhere, and these open wounds are at risk of getting contaminated and eventually infected, which delays healing.
If left unchecked, we have seen globally that 60% of wounds delay in healing just because of an infection. So the idea is, if you can find this infection in an evidence-based way early on, you can treat it early. But you cannot see bacteria and fungus with your naked eye. That’s why we’re using a novel optical biopsy solution.
What is unique about our multispectral imaging solution is that we have identified the different biomarkers that are specific to each bacteria and fungus and integrated these light sources into a portable form factor, and we collect the spectral information through imaging.
On top of this, we apply an artificial-intelligence model trained on close to 2,000 images in India alone, which can compensate for different background noise due to variation in skin color, different chromophores. And it can also tell you the gram type of bacteria, which is clinically relevant.
It provides this information within two minutes, which is like 100× reduction when compared with traditional methods, like a culture test. The solution works for various skin colors as well. The AI runs on the edge, so you don’t need cloud connectivity, especially in a resource-poor area. But at the same time, it has the mechanism to document wounds, monitor the progression of the infection, whether the wound is healing or not—all of that in a noninvasive, portable way.
Is the machine-learning aspect of the technology ongoing—do you continue to train and refine the models?
“We send software updates, just like you receive a new iOS update. In the same way, we have an improvised algorithm in the software that just pings in the hardware that we supply.”—Geethanjali Radhakrishnan
Yes, absolutely. We have now started commercializing in India; we have all the approvals for that. So at the installations that we have, when people agree to participate, we are able to continue to train the model with their data. At different points in time, we try to improve the accuracy of the data that we collect. We send software updates, just like you receive a new iOS update. In the same way, we have an improvised algorithm in the software that just pings in the hardware that we supply. So it’s a continuous learning model.
For example, right now, biofilm is a major cause of concern—when bacteria start forming an extracellular matrix, it makes it even tougher for physicians or anybody from a wound care background. An antibiotic just doesn’t work, you need to do a particular surgical procedure to disrupt these biofilms.
So now we have an ongoing model with our existing data to also detect and classify biofilms from a normal bacteria. This is something that is extremely revolutionary. When such an update goes out, doctors will see more in terms of value proposition in terms of treating a wound than what is available in the market.
How did you get interested in entrepreneurship?
After completing bioengineering, with just an undergraduate degree in it, there are not many opportunities. And typically, people tend to do a masters or maybe a Ph.D. But I did not have the kind of resources to go for higher studies.
Back then, the government in India was promoting entrepreneurship in a very big way, specifically toward novel and innovative ideas, because any kind of innovative solution requires a lot of funding. So they were giving a number of grants. And I did a small immersion program where I went to a hospital, met with a doctor and tried to understand this wound-care space much better in terms of what particular idea I wanted to get into.
And then I pitched for this government grant-funding agency, which is the department of biotechnology grant fund called BIRAC BIG (Biotechnology Ignition Grant). And it didn’t matter whether I was an undergrad or I had a Ph.D.; all that mattered was whether I was addressing a need and had an innovative solution that could really disrupt this particular space.

Radhakrishnan, far left, receives the BIRAC BIG grant. [Image: Courtesy of Adiuvo Diagnostics]
So they were willing to put in the initial amount of grant, which helped me get to a proof-of-concept stage, because it was a lot of establishing basic science about autofluorescence of bacteria and all of that. And that really helped me with founding my company.
Little did I know that it’s not all about science, it’s also about running a business: team management, how to make strategic partnerships. So I think I learned along the way and without knowing much about what I was actually getting into.
Was there anything in your experience that had drawn you to this wound-care idea or to the health care sector?
I was born and brought up in the hills, and for access to health care, you had to travel 100 km to a tertiary hospital. When there are people residing in the hilly region, early diagnosis or screening is very tough. So they always go to a doctor or a tertiary hospital at a very advanced stage, mainly due to the lack of access.
That’s really something that I wanted to work on, which is why I took bioengineering. But I didn’t get many opportunities in it. So I was a software engineer for four years, but I always wanted to get back into doing something for society in terms of health care. Many of the solutions are more customized for the Western population with a different skin color, not particularly to work in India. That’s what really drew me to work on something that my bioengineering skills could be used for; that was the real passion behind it.
As soon as I quit being a software engineer, I realized that I needed to find a problem statement. And that’s when I met with a doctor, Aayush Gupta of DY Patil Hospital in Pune, India, whom I knew. And we saw a number of cases where, for example, a farmer who earns a couple of hundred dollars a month was treated with antibiotics for a fungal infection. It was the wrong treatment, just because there was no point-of-care solution that could tell why it was infected. And he came to a tertiary hospital at a very advanced stage of infection, so the limb had to be amputated.
So that just really moved me. If you have something at a point of care that can tell a nurse whether it is a bacterial or fungal infection, and what stage it is, and connect them immediately with the nearest physician or wound-care professional available, that would save a lot of the patients’ hard-earned money. And they also need the limb because being a farmer, how can they work without a leg?
“Our whole vision is to prevent amputations and misuse of antibiotics.”—Geethanjali Radhakrishnan
So our whole vision is to prevent amputations and misuse of antibiotics. Antimicrobial resistance, unnecessary prescription of antibiotics—it’s a very big thing that’s happening, and there have to be solutions that can minimize the use of unnecessary antibiotics.
What helped you most along the way?
The first is the funding agency, the BIRAC BIG grant, which I spoke about. In terms of mentorship, I was incubated by a social incubator, one of the biggest in India called Villgro, so I had access to a mentor pool.
And one of the very prominent people that I should mention is Arun Venkatesan. He was very important in terms of building frugal innovation. There are different ways of approaching and building a product, but being very frugal, understanding where we may fail in advance, so that we’re able to build a product in a more efficient way—this was very helpful.
And I was a single founder. So it’s very tough—when you go pitch to VCs, they always want to look at how big of a team you have. I was fortunate enough to have a good mentoring network. My current CTO, Bala Pesala, was a mentor back then. So having him from the technology angle and also in terms of product development and industry veterans advising us on commercialization. And from a labs perspective, because all these innovations require a good, well-established facility, we had access to another incubation center called Venture Center, Pune, which is based out of the western part of India, where we did all the microbiology analysis.
So what I realized along my journey, even though I’m a single founder, is that having those mentors at different parts of your product development is very important.
What was it like being a single founder?
I was fortunate enough that my very first few hires were people that I knew before, from my school days or my college days. So it was quite easy to work with the team. I understood that having a good team who can support you, whether it is a failure or a success, is important.
So I try to always have a good team dynamic, and also speak to them very frankly if things are not working, try to understand if something is bothering them. That is also very important if you’re a single founder.
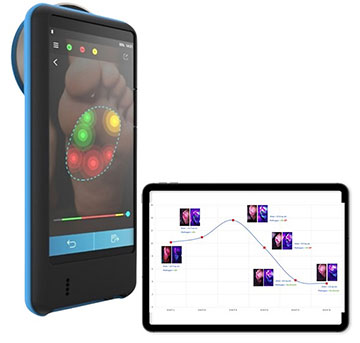
The Illuminate device. [Image: Courtesy of Adiuvo Diagnostics]
Can you comment on the importance of business accelerators and innovation challenges for someone who’s trying to start a new company?
One thing I realized along the way is, of course, we are very resource-poor-setting focused, but we also want to go global. The wound-care problem is pretty common, whether you are in a developing country or a developed country. And being in India, we did not know, for example, how an FDA mechanism works. How do you get approval for the European market? How do you get a Singapore HSA approval?
So it was very important for us to understand that ecosystem and network with like-minded entrepreneurs from that particular segment who are well versed with understanding these regulatory processes, including commercialization.
I attended a number of such challenges and accelerators. One was from SPIE, which is specifically for optics and photonics, and it was very enlightening. I was thinking that my pitch was super-perfect, but when I went there, I was nothing. That really helped me understand the perspective that I had. There was a lot of learning I needed to do in terms of the US market.
And I do attend optics and photonics conferences quite regularly because you need to know the new state-of-the-art technology. I also pitch whatever product we’re working on; we have a scientific talk that I present. So it also helps you network even with big companies, where you find strategic partnerships. So that is very important.
For Singapore, we did the AIPAC entrepreneurship program where you learn about the markets in Singapore and the Asia Pacific region and what are they looking for in terms of investment and other things.
Recently there was also a Boston M2D2 (Massachusetts Medical Device Development Center) impact program that was run by Johnson & Johnson. So that was another eye-opening session. I knew nothing about reimbursement pathways, so I thought, if you get an FDA approval, you’re through. But no, then there needs to be a reimbursement, and for that you need a number of things. That’s how you stay up to date if your focus is to go global.
What are your plans for the global market?
“The US market is especially going to be a focus right now. So we have a clinical advisory board, which consists of both international advisors from the US and Indian advisors.”—Geethanjali Radhakrishnan
The US market is especially going to be a focus right now. So we have a clinical advisory board, which consists of both international advisors from the US and Indian advisors. That was something that we consciously set up as a clinical board team, so that we can move into the US next. Right now we are in the process of getting our FDA approvals. We’re also working on the new EU MDR (Medical Device Regulation), the new CE marking. So we are looking at another one to two years to get both these approvals in place.
We are currently fundraising, and we are looking for a series A round. We have interest from a couple of investors, and we are just trying to close in the next three to six months for this expansion plan.
Are you looking at new products as well beyond Illuminate?
We can use the same multispectral imaging modality along with AI for different applications. We can apply the same device and collect data on, let’s say, cancerous tissue, food, water quality—and we can just tweak the AI to detect these pathogens. We realized it’s very easy for us now to get into all these application areas.
The second thing that we are working on is something called time-dependent fluorescence. Recently my CTO presented on this at SPIE for an invited talk. We are trying to do antibiotic susceptibility patterns within four hours. So if we can actually make this, it would be really game-changing. For time-dependent fluorescence, we have brought down the cost to I think one-tenth of what was out there. So that’s really in terms of technology, what we are working on.
For all these different applications, we have strategic partners who can take them forward, because it’s very tough to commercialize. So it’s better to have a strategic partner who has interest in these specific applications. We develop the technology for them, so that they can take it forward. So we are effectively able to use the available technology that we have developed for these different application areas. That’s also something that we do consciously within our R&D team.
What lessons would you share with someone just starting out to build a company?
At least in India, we don’t take our IP planning or IP portfolio−building very seriously. We never know when we start out what our IP potential could be eight years down the road. So I think if we could plan it up front, that would really be a great boost, especially for deep-tech companies. This could be well known for companies from other areas, but at least coming from India, this is something that I didn’t know when I started. And now I’m realizing what potential it could have.
Another major thing is you cannot be a “jack of everything.” The company takes more than just research and development—you should also have some acumen in terms of understanding sales and marketing. But you should also ask for help, as you may not be the right person to address a particular problem. There are many people who are willing to help you. But if you don’t know when to ask for it, you will never move forward fast.
And last but not least, when you start your company, it’s hard if you don’t understand your team—or rather, if you don’t have watertight agreements in place. When a big investor is coming and looking at you, it will always be better if you have all those agreements in place, even when you first start the company. Have clear demarcations of what is the role of the founder, what is the role of the CTO, what is the role of your key employees. That could help resolve many of the conflicts down the line.
