Caenorhabditis elegans about 1 mm in length. [Image: HeitiPaves / Getty Images]
Neurotransmitters act as chemical messengers that carry signals from neuron to neuron or to other neighboring cells. The brain needs neurotransmitters to regulate essential bodily functions such as heart rate, breathing, muscle movement and sleep.
Now, researchers at ICFO–The Institute of Photonic Sciences, Spain, have discovered a way to control neuronal activity by using photons as neurotransmitters (Nat. Methods, doi: 10.1038/s41592-023-01836-9). The system, called PhAST (photon-assisted synaptic transmission), gives neuroscientists another tool to study the underlying mechanisms of brain function and complex behaviors.
Borrowing from nature
Light is already a crucial part of neuroscience experiments around the world in the form of optogenetics. Developed over a decade ago, optogenetics is a technique that allows for the activation or suppression of neuronal activity by genetically modifying neurons to express light-sensitive ion channels. However, for vertebrates, light needs to be delivered via skull-implanted light sources, and the intensities required to reach deeper brain layers could lead to tissue damage.
The ICFO researchers decided to take a different approach to optogenetics, one that involves sending photons as transmitters between neurons instead of chemicals. PhAST, a genetically encoded system that leverages derivatives of natural light-sensitive ion channels and light-generating enzymes, avoids these experimental drawbacks, including the need for the external light source. The researchers sought out enzymes that are a spectral match to the ion channels, said study author Michael Krieg. Such enzymes—called luciferase—can be naturally found in many animals, he added.
Restoring neuronal communication
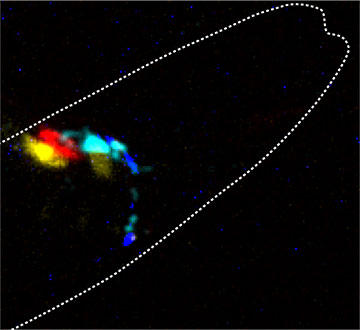
A single worm in which the type of chemical neurotransmitter has been replaced by the color of the photon. The cyan neuron is presynaptic to the yellow and red neurons and thus enables control over their activity using cyan-colored light. [Image: ©ICFO]
To demonstrate the applicability of PhAST, Krieg and his colleagues genetically modified the roundworm Caenorhabditis elegans, a model organism widely used in biological research. In particular, they severed the animals’ nociceptive avoidance circuit, which enables C. elegans to avoid nose touch stimuli. The mutant roundworm was encoded with a luciferase-based optogenetic light source in the presynaptic neuron and a light-sensitive ion channel in the post-synaptic neuron.
To observe the PhAST process at work, the researchers developed a specialized microscopy for highly sensitive photon counting. They paired this technology with artificial intelligence to obtain a clearer picture of the photon emission with synapse-resolution.
The results revealed that photons can indeed act as neurotransmitters. A new synaptic transmission was established between two unconnected neurons, restoring neuronal communication and allowing the mutant roundworm to respond to a nose touch stimulus.
Future applications
In terms of future research, Krieg hopes to build more complex networks in the future, involving more neuronal pairs within a circuit. One potential application for PhAST is for design of synthetic neuronal networks or modulation of dysfunctional synaptic transmissions to alleviate neurological disorders.
“Because all the components can be genetically encoded, PhAST can in principle be applied in any cellular system, including humans,” he said.
