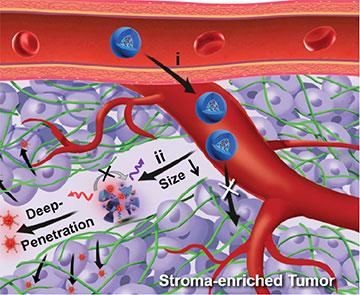
Illustration of nanotheranostics sequence for targeting pancreatic tumor. [Image: JACS Au, doi: 10.1021/jacsau.1c00553, CC BY 4.0] [Enlarge image]
The most common type of pancreatic cancer, pancreatic ductal adenocarcinoma (PDAC), has a five-year survival rate of less than 10%. This deadly cancer is difficult to diagnose and treat in part because the tumors are very dense, preventing imaging dyes and medicines from reaching the cancerous cells packed inside. Now, researchers in China have demonstrated a system for delivering fluorescent nanoparticles throughout these solid tumors that may offer new ways of identifying cancer and delivering targeted therapies (JACS Au, doi: 10.1021/jacsau.1c00553).
In proof-of-concept demonstrations, the engineered nanoparticles were activated by cancer’s acidic environment, penetrated the center of lab-made PDAC tumor models, and emitted strong fluorescence signals for high-resolution imaging of human PDAC tissue samples.
Constructing a theranostic nanoparticle
Led by Hui Li and Zhiqian Guo, the researchers looked to theranostics technology—a combination of therapeutics and diagnostics—to get inside pancreatic tumors. They designed a fluorescent nanotheranostic system called TCM-U11&Cy@P. At the heart of the system is a compact aggregate of molecules, or micelle, consisting of a pH-sensitive copolymer that binds two fluorescing nanoparticles: a cyanine fluorophore (Cy) nanoparticle and a smaller aggregation-induced emission nanoparticle (AIE TCM). The latter is attached to a U11 peptide that targets receptors on PDAC cells.
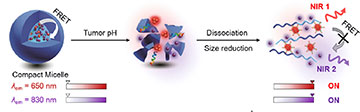
A nanotheranostic compact micelle breaks apart in an acidic cancer environment, releasing fluorescent nanoparticles that can penetrate dense pancreatic ductal tumors. The nanoparticles emit bright near-infrared fluorescence signals and bind to cancer cell receptors, allowing for high-resolution imaging and labeling for targeted tumor ablation. [Image: JACS Au, doi: 10.1021/jacsau.1c00553, CC BY 4.0] [Enlarge image]
In theory, when the compact micelle travels through the bloodstream and reaches the tumor, the copolymer dissolves in the acidic PDAC environment (pH lower than 6.8, compared with a normal body pH of 7.4) and releases the now-activated nanoparticles. The Cy nanoparticle emits a fluorescence signal at 830 nm once released into the tumor environment, and the AIE nanoparticle penetrates deep within the tumor and can be tracked in real time by its 650 nm fluorescence signal. The U11 peptide bonds to cancer cell receptors, marking them for tumor ablation or other targeted therapies.
Demonstrating tumor permeability and in vivo mapping
The researchers used a 1-mm multicell tumor spheroid to simulate a PDAC tumor for permeability demonstrations using their TCM-U11&Cy@P system. After incubating the tumor spheroids with the compact micelle, they recorded 2D fluorescence images at 650 nm and 830 nm every 9 µm throughout the tumor model. The images confirmed that the fluorescent nanoparticles penetrated the center of the ball of cells, and were combined to produce rotating 3D videos of the tumor spheroid.
The researchers incubated TCM-U11&Cy@P with human PDAC tissue samples to demonstrate in vivo tumor mapping. The resulting fluorescence images, taken at both 650 nm and 830 nm, were high-resolution and distinguished pancreatic cancer tissue from normal tissue. The fluorescence imaging results were confirmed with standard histopathology H&E staining of the tissue samples.
Based on their demonstrations, the research team concluded that the TCM-U11&Cy@P system shows promise for improved PDAC detection and targeted treatment and “presents a new visualization strategy for the in vivo application of intelligent nanotheranostics.”