The blood–brain barrier (BBB)—mediated by processes at the “end feet” of specialized nerve cells called astrocytes—protects the brain from harmful substances, but can also keep out medicinal drugs. A team led by researchers at the University of Texas at Dallas, USA, has developed an approach that uses lasers and gold nanoparticles to briefly open a path through the BBB to let drugs through. [Image: Z. Qin / courtesy of the authors]
The blood–brain barrier (BBB) is the brain’s gatekeeper, letting in essential nutrients and keeping out unwanted molecules. Unfortunately, the BBB’s selective nature also keeps out most medicinal drug treatments—an estimated 98% of small-molecular drugs can’t make it into the brain, which severely limits their potency.
Now, researchers have developed a technique that can pry open the BBB’s doors to let in medicine. The method uses light pulses and gold nanoparticles to open blood vessel doorways in the BBB, called tight junctions, for fractions of a second (Nano Lett., doi:10.1021/acs.nanolett.1c02996).
“This new technique has advantages such as high spatiotemporal resolution, reversibility and safety,” said Qi Cai, a mechanical engineering research associate at the University of Texas at Dallas, USA, and a coauthor on the new paper. “We demonstrated that the light-activated increase in BBB permeability does not disrupt the health of the neurovasculature … Thus, it also minimizes the risk of compromising nutrient supply and injury to vulnerable brain tissue.”
Golden treatment
The new technique builds on previous biomedical research with gold nanoparticles. Such nanoparticles can be manipulated with light, which can cause them to heat up nearby water molecules or generate tiny mechanical waves. These effects are thought to have a wide range of uses in diagnostics, imaging and therapeutics.
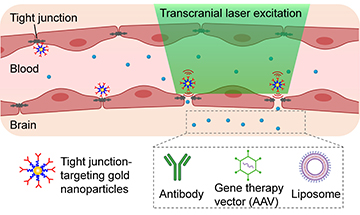
The researchers synthesized gold nanoparticles specifically to target “tight junctions”—doorways in the BBB—and demonstrated that transcranial picosecond laser stimulation of the nanoparticles post-intravenous injection increases the permeability of the BBB. [Image: X. Li et al., Nano Lett., doi: 10.1021/acs.nanolett.1c02996 (2021) / Courtesy of UT Dallas] [Expand image]
A group led by researchers at the University of Texas at Dallas wondered if the same effects could be used to manipulate the BBB. To find out, the team first injected gold nanoparticles into the bloodstreams of mice. Targeting the nanoparticles in the brain with laser pulses lasting one trillionth of a second, the researchers found they could induce the nanoparticles to produce a tiny mechanical force that opened gaps through the specific spots in the BBB.
The effects were short lived—after the laser pulse ceased, the barrier gaps closed with no lasting harmful effects on the BBB, which resumed normal function. But the brief openings were long enough to allow molecules like immunoglobulins, adeno-associated viral vectors (which can be used in gene therapy treatments) and liposomes to find their way into the brain.
Better gatekeeping
Until now, most techniques to get medicine across the BBB affected the whole brain. Ones that did not, including a method using ultrasound, were limited by bone structures. The new technique is not impeded by complex bone structures and can target specific regions. The permeability level can also be adjusted by changing the laser pulse count and intensity and the size of the gold nanoparticles.
The first results give researchers hope that the technique could be used to deliver therapeutic agents for brain ailments such as brain tumors and amyotrophic lateral sclerosis (“Lou Gehrig’s disease”). It could also be used to help with stroke recovery and gene therapy, though more testing is needed before such treatment could be used in human brains.
“The successful delivery of immunoglobulins, adeno-associated viral vectors and liposomes open new avenues for drug screening and therapeutic interventions in the central nervous system,” said Dr. Zhenpeng Qin, an associate professor of mechanical engineering at the University of Texas at Dallas and study coauthor.