In the heterodyne system developed by U.C. Davis engineers for measuring brain blood flow, light shined into the head is returned to a detector, and the signal is boosted by a reference light beam. [Image: Srinivasan lab/U.C. Davis]
A research team at the University of California, Davis (USA), led by engineering professor Vivek Srinivasan, has leveraged interferometry to improve the resolving power of a common technique for assessing blood flow in the brain (Optica, doi: 10.1364/OPTICA.5.000518). The team’s approach—which uses multimode fiber to boost the number of photon-counting channels providing a signal, and which gathers data using a conventional CMOS camera chip—could, according to the researchers, “enhance performance and reduce cost” of existing instruments that rely on the weak, single-photon signal of diffuse optics to assess blood flow in stroke and brain trauma victims.
DCS: Effective but costly
The idea of using fluctuations in scattered light as a practical way of measuring blood flow has been around since the beginning of the decade. The technique, called diffusing wave spectroscopy (DWS) or diffuse correlation spectroscopy (DCS), works by placing an infrared light source at one point on a patient’s skull and a detector at another point a short distance away. Biological tissues, including flowing blood cells, multiply scatter the source light, which in turn is picked up by the detector. Intensity fluctuations in the scattered light, coupled with an appropriate light-transport model, can be used to interpret the dynamics of blood flow in the hidden tissue.
While DCS works, it has some drawbacks. One is that it is a homodyne system—that is, it measures intensity fluctuations tied to a single, multiply scattered light field. As a result, DCS returns a very weak signal that requires costly single-photon counters, such as arrays of avalanche photodiodes, to read.
Improving the signal by boosting the laser power at the source isn’t an option in dealing with live human patients, as ANSI safety standards limit the maximum permissible exposure to 4 mW/mm2. And, while in principle multimode fibers (MMFs) might raise throughput and allow a stronger signal at the collection end, DCS has generally used only single-mode fiber (SMF) or few-mode fiber (FMF), to avoid allowing multiple light modes to muddy the speckle contrast essential for a readable signal.
Leveraging heterodyne detection
Srinivasan’s U.C. Davis group saw an opportunity to improve DCS by leveraging heterodyne detection, and by looking again at the possibilities for MMF in the collection scheme. In theoretical work and subsequent numerical simulations, the team found that, by combining a heterodyne method with an appropriate detector array, it could take advantage of MMF’s better throughput without losing coherence and speckle contrast. That, in turn, could boost the photon budget at the detector sufficiently to eliminate the need for costly single-photon detectors, and allow for much cheaper options—even a non-scientific-grade CMOS camera.

Vivek Srinivasan. [Image: U.C. Davis]
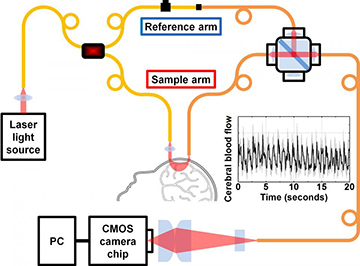
To test the theoretical and numerical results, the team began with an 852-nm distributed Bragg reflector laser tied to an FMF, and split the laser light two fiber channels: a reference arm to serve as a local oscillator in the heterodyne scheme, and a sample arm sent down to couple to the sample (or to a patient’s skull). Roughly 2.5 cm away, another, multimode fiber gathers scattered signal light, and channels the light into a coupler that interferes it with the reference-arm light. A line-scan CMOS camera reads the output from the multimode coupler as an interference pattern.
The interference between the weak scattered signal light and the reference signal yields a 2-D speckle pattern that can be read by the CMOS camera, rather than the sparse data from a homodyne scheme requiring an array of single-photon detectors. And, by carefully modeling the detector geometry for MMF use, the scheme opens up multiple detection channels to further boost the signal, potentially allowing measurements at deeper tissue depths.
From Brownian motion to blood flow
The Srinivasan team tested the setup with a “lipid phantom” solution, at source-detector separations of 1 to 4 mm, and was able to pick up the signal of Brownian motion in the solution. The researchers also performed an experiment to measure blood flow on a healthy human subject, using source and detector fibers aimed at the subject’s forehead but not actually in contact with it, and separated by a distance of 2.5 cm. The setup was able to pick up pulsing blood flows that the team validated by comparison with the signal from a pulse oximeter attached to the subject’s fingertip.
The team believes that the system, which the researchers call interferometric DWS (iDWS), can expand the use of DCS in the clinic by liberating it from reliance on costly single-photon detectors, and instead allowing far cheaper CMOS camera technology that “is rapidly advancing due to mobile phone and autonomous driving applications.” The team is now working to extend tests of the device to a larger population of test subjects, and U.C. Davis has applied for a provisional patent on the technology.
