 [Nanoclustering / Science Photo Library]
[Nanoclustering / Science Photo Library]
In March 2001, California-based Cygnus Inc. received US Food and Drug Administration (FDA) approval for a wearable medical device that seemed poised to revolutionize the way people with diabetes managed their disease. The GlucoWatch Biographer—which looked like a bulky but otherwise ordinary digital wristwatch—claimed to provide frequent, continuous blood glucose measurements for up to 12 hours.
But the product’s most impressive attribute was its ability to do so without drawing any blood. Instead, the GlucoWatch used an electric current to pull interstitial fluid containing glucose molecules to the skin surface, where an electrochemical sensor directly measured the glucose concentration.
Unfortunately, the amount of current required to extract glucose also caused skin irritation, leading to redness, burns and even blisters. In addition, rapid changes in glucose concentration could not be accurately detected with the GlucoWatch, severely limiting its everyday utility. Users were dissatisfied with the device, manufacturing discontinued and Cygnus closed in 2005—a mere four years after its product’s landmark FDA approval.
“Hunting the Deceitful Turkey”
While the GlucoWatch is still the only noninvasive glucose monitor to reach the market, its rapid rise and fall is a fate that has been shared by countless other devices and companies reaching for this particular milestone.
“Noninvasive glucose monitoring is the holy grail of the entire self-diagnostics field,” said Ali K. Yetisen.
“Noninvasive glucose monitoring is the holy grail of the entire self-diagnostics field. Over the last 50 years, there have been significant investments in trying to find a noninvasive sensor for glucose sensing, and all the attempts so far have failed,” said Ali K. Yetisen, a chemical engineering professor at Imperial College London, UK. “People have looked at, for example, Raman spectroscopy, near-infrared (NIR) spectroscopy and many other types—but none of these approaches have been successful.”
Most notably, Apple has wanted to incorporate this feature in its smartwatch for years, having filed a related patent in 2018. Rumors about a possible forthcoming blood glucose sensor have circled the release of every new series of the Apple Watch. The other big tech players—Samsung, Fitbit and Alphabet/Google—have all also shown interest in adding glucose monitoring to their wearables.
“I’ve watched so many companies chase this down. Scientists tend to lose their objectivity when they get infected with [the idea of pursuing] noninvasive glucose measurement,” said John L. Smith, an analytical chemist and consultant who has been involved in the field for 35 years. “I’ve been there. I’ve had the infection.”
In 1998, he retired as the chief scientific officer and vice president of the LifeScan division of Johnson & Johnson, a company that aggressively worked toward a noninvasive glucose sensor for over a decade. In 2006, he decided to document these past failures in a book to warn the field’s newcomers.
“To be quite candid, I don’t see anything that shows any greater promise than I saw 40 years ago, or 20 years ago, right now, in the optical measurement and spectroscopy areas. It has been investigated so many different ways, so many different times,” Smith said. “People walk over the same ground without knowing what the failures were before, and that’s why I went to the trouble of writing a book.”
The Pursuit of Noninvasive Glucose: “Hunting the Deceitful Turkey” is now in its eighth edition, with seemingly no end in sight. Smith updates his tome every two years, hoping each edition will be the last, but as he notes in the latest version, “there has been no decrease in the number of new attempts to end this pursuit.” (The subtitle refers to Mark Twain’s autobiographical short story about chasing a wild turkey that remains just out of reach—“not close enough for success, but just close enough to convince me that I could do it next time.”)
Despite past failures, the demand—as well as the funding—for noninvasive glucose sensing is still as high as ever. Can a new generation of optical technologies finally provide a path to success?
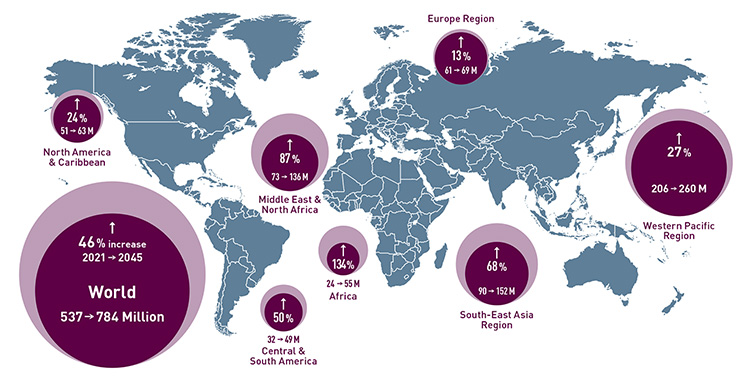 Diabetes around the world: According to the International Diabetes Federation, an estimated 537 million—or one in ten—adults aged 20–79 are living with diabetes worldwide. The number is predicted to rise to 784 million by 2045. [Enlarge graphic] [Source: International Diabetes Federation, 2021 Diabetes Atlas]
Diabetes around the world: According to the International Diabetes Federation, an estimated 537 million—or one in ten—adults aged 20–79 are living with diabetes worldwide. The number is predicted to rise to 784 million by 2045. [Enlarge graphic] [Source: International Diabetes Federation, 2021 Diabetes Atlas]
A fundamental need to monitor glucose
Diabetes is a chronic condition that impairs the body’s regulation and use of glucose as a fuel. Insulin, a pancreatic hormone, helps glucose move from the bloodstream into cells to be used as energy. When the body doesn’t make enough insulin or doesn’t use insulin well, glucose accumulates in the blood, leading to serious health problems such as blindness, kidney failure and stroke.
Patients with diabetes must precisely monitor and carefully control their blood glucose level to keep it within a normal range and prevent complications. Portable finger-prick blood glucometers—which require patients to pierce their fingertip and put a drop of blood onto a test strip for chemical analysis—remain the most common approach to measuring blood glucose. Having to take such tests multiple times per day is inconvenient, painful and impossible to do while sleeping or driving.
“Invasive glucose monitoring means sticking a needle into somebody, pulling out blood and making a measurement. But other methods are considered minimally invasive, where you put a needle into somebody, but it’s not all the way into a blood vessel—it just sits in the skin,” said David Klonoff, medical director of the Mills-Peninsula Medical Center Diabetes Research Institute, USA. “So there is some invasiveness to it, but it … doesn’t hit as many nerves.”
Continuous glucose monitors (CGMs)—available commercially from companies like Medtronic, Dexcom and Abbott—have emerged as a minimally invasive, user-friendly alternative to traditional glucometers. Instead of directly measuring blood glucose levels, CGMs measure glucose concentration in the interstitial fluid via a subcutaneously placed thin electrode coated with a glucose-sensitive enzyme.
However, “even though [CGMs] are a large commercial success, still there is room to improve the technology,” said Jeon Woong Kang, research scientist at the Laser Biomedical Research Center at the Massachusetts Institute of Technology (MIT), USA. “A minimally invasive sensor should be replaced every one to two weeks, and since it uses an embedded wire under your skin, it can cause skin irritation and sometimes inflammation. Also, the accuracy of a minimally invasive sensor is not quite as [high] as the finger-stick device.”
The appeal of a noninvasive glucose sensor is obvious—aside from the convenience, there is no pain, no blood and no risk of infection.
The promise of “no more finger pricks”
The appeal of a noninvasive glucose sensor is obvious—aside from the convenience, there is no pain, no blood and no risk of infection. So what makes building an effective one so difficult? And why have optical approaches failed up to this point?
In the early 1990s, optical studies demonstrated strong correlations between blood glucose concentrations and the intensity of light reflected from skin during oral glucose tolerance tests, in which subjects would ingest a sugary drink between blood draws. Later, it was determined that the results were due to changes in the refractive index of skin—a variable affected by just about anything, including albumin, organelles, osmotic pressure and temperature.
After realizing that specificity for glucose is essential, researchers moved to NIR and Raman spectroscopy involving glucose-specific optical signatures that stand out in comparison to other chemical components found in skin tissue. But each spectroscopic technique has its own set of challenges.
NIR spectroscopy has the advantages of simple and relatively low-cost instrumentation and greater penetration depth (greater than 1 mm) compared to other wavelength ranges. The technique has perhaps been the most frequently attempted route for noninvasive glucose monitoring—meaning that it has failed just as many times.
Glucose’s several NIR absorption peaks are broad and weak, and they overlap with other interferences, such as water or lipids. Environmental factors like temperature and humidity may also confound results. Finally, there are likely hundreds of other compounds in the body that have a structure close to that of glucose with comparable spectral features.
“If I look at a spectrum of glucose, lactate and albumin as examples, they will be unique in shape, but they will be overlapping to the point that you couldn’t pick a single wavelength to look at glucose relative to the others, or a wavelength for the others to measure relative to glucose,” said Mark Arnold, a laser chemistry professor at the University of Iowa, USA. “You’d have to have a full spectrum in order to pull out selectivity based upon on spectral orthogonality.”
Unlike glucose’s NIR absorption spectrum, its Raman spectrum can be clearly distinguished from those of other biological compounds. However, the Raman signal is very weak, so the signal-to-noise ratio tends to be a problem. Devices have used long acquisition times as a result, which can make the measurement sensitive to laser intensity fluctuations.
“Raman spectroscopy has a huge benefit in that it’s much less sensitive to water than near-infrared spectroscopy … Plus, they can get a first-order spectrum of glucose or these other molecules because of that insensitivity to water,” said Arnold. “One of the issues with Raman spectroscopy, at least in my view, is that it is a scattering method, and scattering methods are inherently very sensitive to experimental parameters that tend to be difficult to control.”
Despite these issues and many others, researchers continue to find new avenues to pursue glucose sensing using light. The following sections highlight three examples of recent efforts.
Moving into the mid-infrared
In 2001, biophysicist Werner Mäntele created a prototype device that used infrared spectroscopy to determine the ingredients of a beer in less than a minute. While it never caught on with breweries, the technology has found an unexpected second life as a noninvasive glucose monitor (see “From Lab to Clinic,” OPN, April 2022).
DiaMonTech, a Germany-based startup founded in 2015 by Mäntele and three partners, has developed multiple devices to measure interstitial glucose with photothermal spectroscopy in the mid-IR spectral region. Mäntele believes that the mid-IR range can provide a path to success, unlike NIR-based methods.
“In our view, the many attempts to use optical techniques failed because, for the sake of simplicity, NIR wavelengths were used. [One reason is that] NIR can detect glucose overtones, but overtones are around 10 to 100 times weaker than the mid-IR fundamental modes,” said Mäntele, chief scientific officer at DiaMonTech, whose company name is a portmanteau of “diabetes monitoring technology.” “NIR thus will not solve the problem, nor lead to the ‘holy grail’ of noninvasive glucose monitoring.”
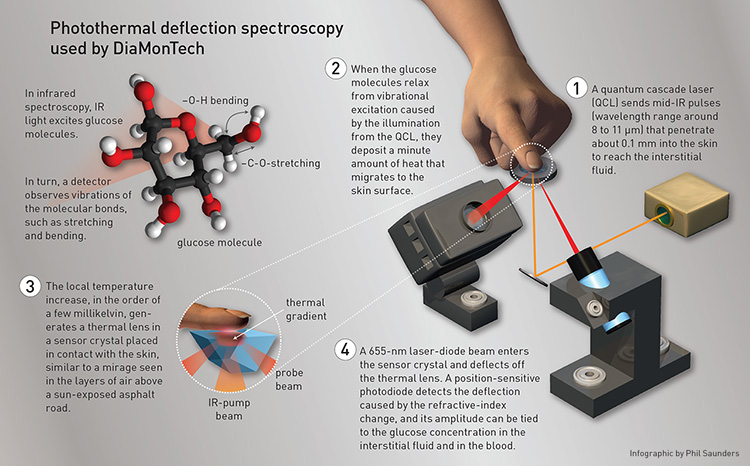 [Enlarge graphic] [Infographic by Phil Saunders]
[Enlarge graphic] [Infographic by Phil Saunders]
The company’s original product, D-Base, is a CE-certified, shoebox-sized table-top medical device meant for use in doctors’ offices, nursing homes and clinics. The company has also applied for FDA clearance. In 2021, Mäntele and his colleagues reported on a clinical study where they tested D-Base on 41 people with diabetes and 59 people without. The results, published in the Journal of Diabetes Science and Technology, showed that D-Base is comparable in accuracy to minimally invasive glucometers and finger-stick devices.
 DiaMonTech’s D-Pocket prototype. [DiaMonTech]
DiaMonTech’s D-Pocket prototype. [DiaMonTech]
Currently, DiaMonTech is developing D-Pocket, a miniaturized version of D-Base, designed to be a personal device about the size of a smartphone. The device is expected to be complete by the end of 2022, with CE approval taking another half-year or so. One battery charge will provide users with approximately 50 glucose measurements.
“D-Pocket will be the daily companion of these patients. Depending on how critical the control is, the patient will set a vibration alarm that reminds [them] to perform a measurement that takes less than 30 seconds,” Mäntele said. “No pain, no discomfort, no blood.”
DiaMonTech is also working with a large Korean mobile phone company to further its technology with integrated optics and miniaturization of the quantum cascade laser (QCL). The resulting product, D-Sensor, will combine the measurement of glucose with others such as blood pressure and heart rate. Unlike D-Base and D-Pocket, D-Sensor will be a continuous monitor, automatically performing a measurement every few minutes and relaying the data to the user’s smartphone.
Observing the glucose fingerprint
For over two decades, researchers at MIT’s Laser Biomedical Research Center have chipped away at the problem of noninvasive glucose sensing, with Raman spectroscopy as their chosen method of attack.
While Raman spectra offer molecular specificity, however, they aren’t without practical limitations. In particular, glucose-specific peaks in transdermal Raman spectra were too weak to be deemed reliable. “First, we couldn’t see the pure glucose signal from the in vivo spectrum. And second of all, because we don’t see the clear signal, there has been a lot of heavy computations needed for the prediction,” said Kang. “Because of that, the prediction model was sensitive to the environment, and prospective prediction was not really possible.”
Continual upgrades of lasers, detectors and electronics over the last 20 years somewhat improved the measurement, but the biggest gain came from a change in the system’s geometry. Previous Raman approaches focused on increasing collection efficiency while maintaining an in-line geometry, which leads to a significant amount of unwanted reflected signal from the skin’s surface.
“Even though we have low-pass filters at the tip of the collection optics, if you have too many reflected photons, some of them are actually passing through and generating background signal during the collection,” said Kang. “And when you have increased background signal, then it means that you have increased shot noise, and those small glucose peaks are easily dominated.”
Instead, Kang and colleagues employed off-axis illumination, where an 830-nm laser was focused on the skin with an incidence angle of 60 degrees and collected by an optical fiber bundle set perpendicular to the surface. Their 2020 Science Advances paper reported the first direct observation of glucose Raman peaks from in vivo skin using this novel geometry.
 A wrist-strap apparatus that can read the glucose level of a user, developed by Jeon Woong Kang and his colleagues. [J.W. Kang]
A wrist-strap apparatus that can read the glucose level of a user, developed by Jeon Woong Kang and his colleagues. [J.W. Kang]
When glucose was intravenously infused to raise live pigs’ blood glucose levels above baseline, the researchers observed a clear glucose signal with intensities that varied proportional to the reference. Although pigs were used, the researchers believe it represented a major advance. They are now working on miniaturizing the device—currently laboratory-cart sized—and testing it on people with diabetes in collaboration with clinicians.
“What we are hoping to see in the longer term is a wearable device with an insulin pump and our spectroscopic device in one package, maybe a little bit bigger than a cell phone, with some linkage to the skin for both the insulin control as well as the spectroscopy detection,” said Peter So, director of MIT’s Laser Biomedical Research Center.
The silicon photonics edge
One company making waves throughout the wearable-tech industry is Rockley Photonics, a global integrated-optics supplier that specializes in health monitoring and communications solutions. Last year, Rockley went public, and a US Securities and Exchange Commission filing revealed Apple to be its single largest customer. News outlets speculated that the tech giant planned to use Rockley’s optical biosensors in future iterations of its smartwatch.
While Rockley representatives won’t comment on this point, the company did confirm that it is working on a noninvasive biosensing platform to monitor multiple biomarkers. The first iteration of the platform, Baseline, will focus on the optical measurement of core body temperature and hydration level. The next generation, Pro, will add alcohol, glucose and lactate sensing. Both the consumer electronics and health-care versions of the product—named VitalSpex and Bioptx, respectively—are worn as compact wristbands.
 Rockley Photonics’ biosensing wristband. [Rockley Photonics]
Rockley Photonics’ biosensing wristband. [Rockley Photonics]
“Our motivation is more than just tracking glucose. We believe that monitoring glucose noninvasively is only one piece of the puzzle and that monitoring multiple biomarkers will provide a more complete view of each person’s health,” said Ben Ver Steeg of Rockley Photonics’ sensing product development. “Our unique silicon photonics technology is able to put this class of sensor into a wearable system for the first time.”
Silicon photonics, the optical analog of silicon microelectronics, employs traditional semiconductor fabrication techniques to produce lasers, photodetectors and other components. The resulting silicon photonic integrated circuits boast compact size, low manufacturing cost, improved scalability and uniformity.
Both VitalSpex and Bioptx contain a “spectro-photometer-on-a-chip,” which integrates distributed Bragg reflector lasers and complex optical circuitry that generate numerous discrete, narrow-linewidth laser wavelengths across a wide spectral range. Light from multiple infrared-region lasers penetrate the skin at varying depths, reaching tissue layers containing biomarkers in interstitial fluid, and are combined to produce a high-fidelity spectral measurement.
“Rockley has the vertical integration in-house capabilities required to design and produce the silicon photonics, the mixed signal [integrated circuit] and the integrated sensor design—all the way to a final wearable product. Our proprietary algorithms process the spectral information to extract the biomarkers of interest,” said Ver Steeg. “We believe that our platform is the only system to date that has this level of performance and breadth of capability in a wearable form factor.”
The company has released white papers featuring results of preliminary human studies for core body temperature and hydration level, which demonstrated good accuracy, but not yet for glucose or the other Pro-level biomarkers. In the short term, Rockley expects to develop its glucose sensor for wellness tracking rather than medical treatment, with the hope of later providing diabetes patients with an FDA-approved version.
Only time—and robust clinical data—will tell whether any of these ventures have a real chance at success.
What the future holds
Only time—and robust clinical data—will tell whether any of these ventures have a real chance at success. Other optical techniques being investigated include optical coherence tomography, photoacoustic spectroscopy and far-infrared spectroscopy. Artificial-intelligence methods are also being applied to optical datasets with a glucose-specific signature to improve clinical performance and measurement accuracy.
“I think it’s a very exciting time for optics for these kinds of measurements. It’s coming about because there’s a lot of venture capital that’s being invested into this space, and that’s generating a lot of innovation, both in terms of the optics and in terms of artificial-intelligence approaches,” said Arnold, who along with Klonoff is a consultant for Rockley. “If noninvasive sensing is going to be successful, optics is going to have to play a key part.”
Others remain more skeptical. Smith has his doubts that optics—or any other approach—will ever attain the original holy grail of an FDA-approved noninvasive medical device for the treatment of diabetes.
“There are so many sources of interference, and the interfering signals are so much greater than the one you’re looking for here, that it is a very challenging pursuit,” said Smith. “I tell everybody, my goal is to see this thing solved during my lifetime. But I don’t know how many more years I have left.”
Steven LeBoeuf, president and co-founder of Valencell, which licenses biometric sensor technology to Samsung, Sony and other companies, has called it “completely impossible” to create a noninvasive glucose monitor.
“We investigated it years ago and, from time to time, we reinvestigate that opportunity with a noninvasive means. We don’t believe it’s possible to develop a truly noninvasive glucose monitoring solution that will be good enough to dose insulin for the foreseeable future,” said LeBoeuf. “However, we do believe it’s possible with a truly noninvasive technology to categorize the blood sugar range you might be in—whether you’re into low range, normal or high.”
Several experts mentioned that setting a lower bar for noninvasive glucose monitoring in this way would be a more realistic goal. People without diabetes are still interested in glucose tracking for wellness and sports, and optical sensors could possibly fill this niche.
Meeri Kim is a freelance science journalist based in Los Angeles, CA, USA
For references and resources, visit: optica-opn.org/link/glucose-monitoring.
