Interactive two-person fNIRS neuroimaging setup from NIRx Medical Technologies. [NIRx]
Interactive two-person fNIRS neuroimaging setup from NIRx Medical Technologies. [NIRx]
Biomedical engineer Frans Jöbsis was preparing a chuck roast for dinner—what he called “the poor academic’s substitute for steak”—when he realized the slab of meat could serve as the subject for a quick-and-dirty experiment. As a professor at Duke University, USA, he was preparing a grant application to study the exposed heart with near-infrared spectrophotometry. Since he had only used visible light at that point, Jöbsis wanted to know how far near-infrared photons would travel through tissue and whether they could pass through bone.
He recruited his teenage son as a laboratory assistant, and the two held the cleaned bone up against a light source. They noticed that the shadow of a finger could easily be seen in the diffuse red light coming through the bone, which was roughly the thickness of a human skull. He concluded that, if red light could pass through bone, then near-infrared light with its longer wavelengths could penetrate through the skull to access the brain.
Today, the most cutting-edge fNIRS systems are wearable, wireless and portable, akin to a swim cap or bike helmet that can measure real-time oxygenation of the brain.
This pivotal discovery in December 1976 led to a shift in his laboratory’s focus that culminated in a very personal experiment––Jöbsis performed transillumination spectroscopy with fiber optics on his own head, shining near-infrared light into one temple and counting the photons leaving the other. During hyperventilation, he successfully recorded a signal change interpreted as the expected drop in blood volume of the brain tissue.
His encouraging results—documented in a 1977 Science paper that has been cited more than 4,200 times—sparked the emergence of a new field called near-infrared spectroscopy (NIRS). Today, the experimental and clinical tool takes advantage of the transparency of skin and bone to near-infrared light to offer noninvasive, in vivo monitoring of tissue oxygenation.
One of the fastest growing areas of NIRS in recent years is functional NIRS (fNIRS), a neuroimaging technology that uses multiple near-infrared light sources and detectors on the head to map activation of the human brain. Today, the most cutting-edge fNIRS systems are wearable, wireless and portable, akin to a swim cap or bike helmet that can measure real-time oxygenation of the brain a hundred or more times per second.
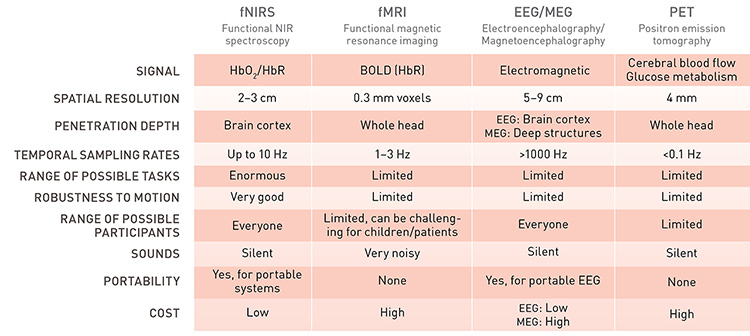 Comparison of fNIRS with other neuroimaging modalities [Adapted from P. Pinti, et al. Ann. N.Y. Acad. Sci. 1464, 5 (2020)] [Enlarge image]
Comparison of fNIRS with other neuroimaging modalities [Adapted from P. Pinti, et al. Ann. N.Y. Acad. Sci. 1464, 5 (2020)] [Enlarge image]
Finding a niche
Since the early 1990s, fNIRS instrumentation has improved from single-channel measurements with one source-detector pair to high-density systems with hundreds of channels that cover the entire head. Such rapid advancements have caught the attention of researchers outside the field who are eager to study the human brain in conditions more natural than the confines of a magnetic resonance imaging (MRI) scanner.
“With an MRI scanner, a person is scrunched horizontally in a tube that is banging on their head at about 110 decibels, and then we ask them to do things,” said Joy Hirsch, professor of psychiatry at Yale University, USA. “It’s amazing how much we understand about neural processes in the brain based on that technology, but if you’re a fly on the wall looking down on it, it’s actually quite laughable.”
Hirsch spent most of her career as a leading pioneer of functional MRI (fMRI) at Columbia University, USA, just to resign in 2014 to begin a new chapter of social-neuroscience research with fNIRS at Yale. Her lab began to experiment with a technique called hyperscanning, where signals are recorded from two or more participants simultaneously to test for the presence of neural circuitry in the brain specialized to the task of interpersonal interaction.
 Interactive two-person neuroimaging setup at Joy Hirsch’s fNIRS hyperscanning lab at Yale University, USA. The purpose of the fNIRS setup is to understand the neural mechanisms that detect, encode and transmit “natural” social cues. [Hirsch Brain Function Laboratory, Yale University]
Interactive two-person neuroimaging setup at Joy Hirsch’s fNIRS hyperscanning lab at Yale University, USA. The purpose of the fNIRS setup is to understand the neural mechanisms that detect, encode and transmit “natural” social cues. [Hirsch Brain Function Laboratory, Yale University]
“I worked in fMRI for a decade and could see that there was a whole domain of questions regarding two-person interactions that we couldn’t get at,” said Hirsch. “It was time to move on—to understand our brain doing probably one of the most important functions in our lives other than breathing, which is connecting with other people.”
For Hirsch and other brain researchers, fNIRS fills a gap for performing neuroscience experiments in situations not feasible to do in an MRI machine. fNIRS experiments have recorded the brain activity of people walking, driving, playing an instrument, speaking to one another face-to-face and playing a communal game, to name but a few. The optical technology is also frequently used to study children, vulnerable patients, claustrophobic individuals and other populations difficult to scan with MRI.
“fNIRS has found a niche, and it’s actually quite exciting to see how the field has developed over the last few years,” said Robert Cooper, fNIRS researcher at University College London (UCL), U.K. “Since I became a part of it back in 2007, it’s really grown significantly, and most of that growth is on the back of studying these types of things that just aren’t accessible with other methodologies.”
The early days of NIRS
At its most basic, NIRS involves sending near-infrared light into tissue and measuring the intensity of re-emerging light. The light will either be scattered or absorbed by color-containing compounds like water, lipids or proteins. While scattering is about 100 times more likely, any absorption that does occur is dominated by hemoglobin, the protein that carries oxygen in blood. When hemoglobin has oxygen in tow, its absorption spectrum differs from that of deoxygenated hemoglobin.
Out of the brains of babes
Researchers at University College London, U.K., captured infant brain activity with a wearable, baby-friendly cap containing 12 hexagonal sensor tiles (left) equipped with three dual-wavelength LED sources and four photodiode detectors. The tiles fit onto “docks” (center) that snap into the cap and provide power and data transfer. A lightguide piece (right) containing short plastic optical fibers couples light through the dock to and from the scalp.
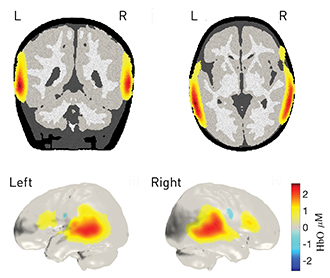 The brain images show changes in the concentration of oxygenated blood in the infant brain in response to human vocal sounds.
The brain images show changes in the concentration of oxygenated blood in the infant brain in response to human vocal sounds.
[DOT-HUB, University College London and Gowerlabs Ltd.]
NIRS leverages this difference by measuring the absorption of different wavelengths to calculate changes in the concentrations of oxyhemoglobin and deoxyhemoglobin. This simple type of NIRS, called continuous-wave (CW) NIRS, can only track trends and does not give absolute numbers.
Jöbsis built the first prototype of a commercial, bedside CW NIRS instrument with his colleagues in 1980, and subsequent development, both in the U.S. and among research groups in Italy, the U.K. and Japan, continued over the next decade. Commercial NIRS systems have since found a place in operating rooms during vascular or cardiac surgery, as well as in pediatric intensive care units.
Yet despite the early excitement, NIRS is not as widely used in hospitals today as proponents of the technology may have initially hoped. Monitoring patients with NIRS hasn’t always demonstrated a clear improvement in outcomes, and the lack of significant results has slowed the adoption of the technology by clinicians.
“There are centers that use commercial NIRS devices and use them quite aggressively, particularly in Europe,” said Keith St. Lawrence, associate professor of medical biophysics at the University of Western Ontario in Canada. “But there’s no indication for clinical value for NIRS. If we can demonstrate a benefit, then it will become more routine, and that’s obviously the hurdle that needs to be overcome.”
A window into the brain
While many research groups still focus on finding clinically useful aspects of the technique, others have branched out into the development and promotion of NIRS as an experimental brain-monitoring tool in the same vein as fMRI.
Even though its clinical applications have remained fairly limited, fMRI has had a tremendous impact in cognitive neuroscience. The idea that MRI could be used to investigate brain activity as well as anatomy is relatively new, with the first fMRI studies appearing in the early 1990s, some twenty-odd years after the invention of the MRI machine itself. Its inception kicked off a scanning revolution, as researchers could peer inside a dynamic, living human brain as never before.
“Before functional MRI, the brain was just a black box. You could poke it, and it would jump, but you didn’t know what went on inside,” said Hirsch. “But we solved the occlusion problem—that is, we could look inside the brain noninvasively in typical human beings and watch it work. I mean, what a gift for a neuroscientist.”
Instead of the greyscale anatomical images of traditional MRI, fMRI researchers now showed off flashy, colorful maps of brain activation during a particular task. They are generated by having subjects alternate between periods of doing a task and a control state, repeated over many trials. The difference between the signal during the task versus control state is taken as activation due to the stimulus.
Like fNIRS, fMRI does not directly measure neuronal activity and instead relies on its coupling with blood flow. When neurons in the visual cortex fire in response to a stimulus, the arteries in that region dilate, leading to an increase in blood flow to support the increased neuronal demand for nutrients. This process, known as a hemodynamic response, manifests as an increase in oxyhemoglobin and a decrease in deoxyhemoglobin.
Over the years, a steady increase in the number of channels has led to the simultaneous measurement of brain oxygenation from anywhere on the head.
The first and most common fMRI signal—the blood-oxygenation-level-dependent (BOLD) contrast—relies on changes in deoxyhemoglobin, which acts as an endogenous paramagnetic contrast agent. In the early 1990s, NIRS researchers began to toy around with the idea of performing functional studies with their instruments, which already output both oxyhemoglobin and deoxyhemoglobin data.
The first handful of fNIRS studies were simple feasibility experiments that focused on single-channel measurements of, for instance, the prefrontal cortex during a calculation task or the visual cortex during visual stimulation. But quickly, researchers realized the need to measure different regions of the brain at once to produce an fMRI-like map of cortical oxygenation.
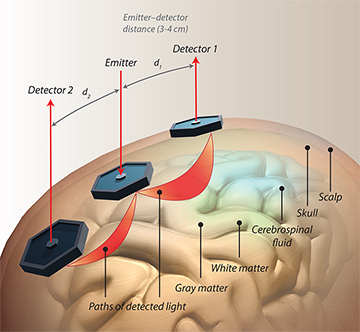 Illustration of an fNIRS setup: NIR photons follow a path (shown in red) from the light source to the detector through the different layers of the head. The penetration depth of the light is proportional to the source–detector distance. [Illustration by Phil Saunders] [Enlarge image]
Illustration of an fNIRS setup: NIR photons follow a path (shown in red) from the light source to the detector through the different layers of the head. The penetration depth of the light is proportional to the source–detector distance. [Illustration by Phil Saunders] [Enlarge image]
Mapping brain activation
Over the years, a steady increase in the number of channels has led to the simultaneous measurement of brain oxygenation from anywhere on the head. For instance, the latest version of NIRScout, a commercial CW NIRS device from the New York-based company NIRx, can have up to 64 laser sources and 32 detectors for a total of 2,048 maximum channels of data. Two systems can even be connected in tandem to double the amount of coverage on a single head.
With more channels available, fNIRS researchers were able to first create 2D topography maps of cortical activity and finally image reconstructions in three dimensions with diffuse optical tomography (DOT). Performing DOT requires a more complex system, with probe configurations that allow for several different distances between sources and detectors.
“DOT came about originally because, instead of just getting channel-wise measurements, people wanted to actually get three-dimensional volume images,” said Cooper. “With DOT, you have multiple measurements packed into a given area of the scalp, and you’re trying to extract spatial information from that data. The measurements are exactly the same as traditional fNIRS, but you’re using overlapping spatial sampling of an object in order to obtain three-dimensional distributions of optical properties.”
The spatial resolution of fNIRS has certainly improved since the inception of DOT but remains much lower than fMRI—around 2 cm to 3 cm for most fNIRS systems, with high-density DOT achieving 5 mm to 10 mm, versus millimeter to submillimeter values for BOLD fMRI ranges in high-resolution adult brain scans. However, fNIRS—with a maximum sampling rate of 100 Hz or higher—has a better temporal resolution than fMRI, which has a sampling rate of 1 Hz to 3 Hz.
Another major disadvantage of fNIRS is a lack of access to deeper regions of the brain. For the adult brain, it can only provide measurements from the surface of the cortex. Increasing the source–detector separation can increase penetration depth, but at the expense of signal-to-noise ratio. Therefore, researchers aiming to study activity in other parts of the brain must resort to fMRI.
Lastly, hair—particularly dark-colored hair—can generate a low signal-to-noise ratio due to its high absorption of NIR light. “Hair is an annoyance to anyone doing human neuroimaging with optics,” said Cooper. However, “It’s not an insurmountable problem.” Typically, strands of hair are moved aside for better optode placement and direct contact with the scalp.
Cooper is founder of the DOT-HUB at UCL, a research group focused on the advancement of DOT for the human brain. In November 2020, he published with his colleagues the first demonstration of a wearable, high-density DOT device called LUMO in infants. They were able to generate high-quality, functional 3D images of brain activation from six-month-old infants, who tolerated the swim-cap–like headgear very well.
LUMO, developed by UCL spinoff company Gowerlabs Ltd., U.K., is part of a new generation of fNIRS technology that is wearable, wireless, miniaturized and modular by design. That means no more bulky, heavy fiber optic cables tugging on the probe caps and restricting subjects’ movement. Tile-like modules containing light sources and detectors in a hexagonal array connect to a headpiece, giving researchers experimental flexibility. And of course, a smaller and lighter headpiece allows for greater comfort and wearability for extended measurements.
Measuring time of flight
A flashy newcomer on the scene that embodies this next-generation approach is Los Angeles-based Kernel, which differs from other fNIRS companies in that it was founded by an entrepreneur and venture capitalist rather than a scientist already working in the field. Founder and CEO Bryan Johnson sold his payments company Braintree to PayPal for US$800 million in 2013 and is now betting US$54 million of his own money on fNIRS as the next big thing in neuroscience. Kernel has also received an additional US$54 million in venture financing from outside investors.
This stark difference in funding availability compared with academia has allowed Kernel to build a sleek, sophisticated product with impressive specifications in just four years. Kernel Flow is a whole-head, wearable fNIRS system that looks similar to a bike helmet, with more than 1,000 source–detector pairs and a 200 Hz sampling frequency. Most notably, it uses a more sophisticated—although by no means new—technique called time-domain (TD) NIRS that yields more information than CW NIRS at the expense of greater instrumentation complexity.
 Kernel CEO Bryan Johnson (left), Kernel Flow headwear (center) and activation map from fNIRS, showing activation for a working memory task on a single participant’s fifth day of a 15-day study (right). [Kernel]
Kernel CEO Bryan Johnson (left), Kernel Flow headwear (center) and activation map from fNIRS, showing activation for a working memory task on a single participant’s fifth day of a 15-day study (right). [Kernel]
Unlike CW NIRS, TD NIRS uses a pulsed laser to capture the arrival-time distribution of scattered photons for each pulse. This temporal spread function of the re-emerging photons provides information on the scattered and absorbed light, as well as the depth reached by the photons within the brain.
“The biggest difference between us and those sort of research-grade TD fNIRS systems is that we designed everything from scratch in a way that was intended to be scalable,” said Ryan Field, CTO at Kernel. “Instead of just optimizing for performance, we tried to design a hardware system that was still useful and does almost as much as these research-grade systems but can become much more widespread and used throughout the community.”
Like LUMO, Kernel Flow was built as a modular system with hexagonal tiles that snap into the helmet. Whole-head coverage requires 52 modules, each with a single pulsed laser and six detectors. The device fits a single TD fNIRS channel on a chip smaller than 2.5 mm by 3 mm.
As a next step, Kernel is partnering with 50 researchers in both academia and industry—many of whom already specialize in or have experience with fNIRS—and giving them a system to test-drive in experiments of their own. For instance, St. Lawrence hopes to receive his Flow system later this year to study the accuracy of coma prognosis and measure whole-brain blood flow during cardiac surgery.
“We had the option of using all 50 of these systems ourselves or open things up to the larger community and start to build an ecosystem where we can standardize hardware platforms across a lot of labs, get people collecting data in the same way, and really amplify the impact of our systems and how they are used,” said Field. “We didn’t want to sell systems to just anyone. Because they’re limited, we wanted to make sure that we got systems in the hands of people who are going to do interesting things with them.”
A growing interest
Similar to Hirsch, St. Lawrence started out in the MRI world and received his training in magnetic resonance physics before diving into NIRS research around 15 years ago. He found himself lured in by the technology’s low cost, portability and safety—and believes that others outside of the optics community must follow in his footsteps if fNIRS is to succeed.
“I would hate to see fNIRS trapped in a world of just people who really know the optics and can do a few studies on it. Because, of course, we’re not neuroscientists,” said St. Lawrence. “We need to have people outside the field involved.”
fNIRS does have inherent disadvantages that hinder its growth—low spatial resolution, inability to measure the deeper brain—but these may be outweighed by its many advantages.
fNIRS does have inherent disadvantages that hinder its growth—low spatial resolution, an inability to measure the deeper brain, the difficulties of dealing with hair—but researchers like Hirsch claim that these are outweighed by its many advantages. Further improvements in technology like high-density DOT, miniaturization and wearability, as well as a greater adoption of TD fNIRS should increase the reliability of measurements and number of applications.
Awareness of and interest in the field is undoubtedly growing, even though the technology itself is still finding its footing. Kernel, for instance, has introduced fNIRS to the tech world and aims to one day take its Flow device from the lab to the home in the form of a daily-use consumer electronics product. A promotional video from the company shows CEO Johnson relaxing at home with Flow on his head acting as a smart assistant to suggest entertainment, food and activity choices based on his current brain activity.
Others like Cooper are more skeptical about consumer-oriented applications, given that the hemodynamic response takes a few seconds to occur, and feel that electroencephalography (EEG) would be a better-suited modality. But whether fNIRS stays in the lab of neuroscientists or makes the leap into our homes, one thing is for certain: Probing the inner workings of the brain with light will continue to get easier, cheaper, faster and more comfortable.
“The brain is kind of a stranger to us compared to things like our heart, lungs or intestines. Finding ways in which we can encode signals from the brain to understand how it operates is forward-thinking,” said Hirsch. “It’s a little bit of a haze out there, but I like that because out of chaos, out of haze, there’s opportunity for new ideas.”
Meeri Kim is a freelance science journalist based in Los Angeles, CA, USA.
For references and resources, visit: www.osa-opn.org/link/fnirs-brain-imaging.