Scatterings
Did You Know?
Insulating compound formed by adding hydrogen to graphene.
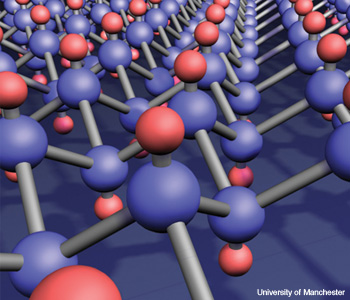 Graphane is an insulating compound formed by adding hydrogen to graphene.
Graphane is an insulating compound formed by adding hydrogen to graphene.
Hydrogenation—the chemical reaction that results from the addition of hydrogen—is widely used in the chemical industries. Margarine may be the best-known example. (Hydrogenation turns liquid fat into solid.) Now, D.C. Elias and others at the University of Manchester in England have reported hydrogenating graphene, a single-atom-thick carbon crystal that was first reported in 2004 (Science 323, 610). Graphene has a fascinating energy structure that lends itself to applications in photonics and electronics.
…Log in or become a member to view the full text of this article.
This article may be available for purchase via the search at Optica Publishing Group.
Optica Members get the full text of Optics & Photonics News, plus a variety of other member benefits.
