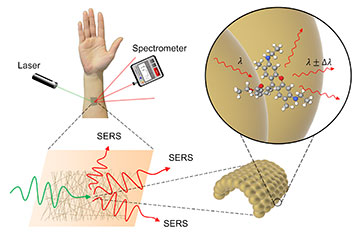
Surface enhanced Raman spectroscopy (SERS) is a method of detecting the presence of a chemical indirectly by using laser light and a specialized sensor. Gold mesh provides an ideal surface for taking measurements, as it does not interfere with the substance being measured. [Image: ©2022 Goda et al.] [Enlarge image]
Over the last 20 years, scientists have raced to develop flexible, wearable sensors that can pick up traces of important biomarkers. One factor limiting the use of such sensors, however, is their inability to detect more than one trace analyte at a time without expensive, bulky modifications.
An international team of researchers has recently improved its flexible biosensor by turning the sensor to gold. Specifically, they designed a finely spun gold nanomesh patch that stretches as the wearer moves and permits label-free spectroscopic detection of multiple chemicals—from urea to frequently abused drugs—in both low and high concentrations (Adv. Opt. Mater., doi: 10.1002/adom.202200054).
SERS and stick-ons
The notion of wearable sensors for multiple biomarkers has received a boost from the development of surface-enhanced Raman scattering (SERS). In the 1970s, scientists discovered that the signal for inelastic light scattering off molecules adsorbed onto corrugated surfaces would multiply by many orders of magnitude. Although whether the SERS effect relies on surface plasmons or charge transfer remains an area of research, biochemists and biophysicists are forging ahead with potential medical applications.
Previous designs for wearable SERS sensors have consisted of multiple layers of nanowire arrays, pillars of gold nanoparticles, or silver nanowires embedded on a film made of silk protein. But Keisuke Goda, a chemistry professor at the University of Tokyo, Japan, and his colleagues deemed such sensors too difficult to manufacture or too limited in their ability to detect unknown substances.
Goda and colleagues found that a group of researchers based in Japan and the Republic of Korea had devised a gold-coated polyvinyl alcohol (PVA) nanofiber that was gas-permeable and stretchable. Its creators developed it by spinning the PVA into a mesh of randomly ordered fibers, depositing gold on the fibers through thermal evaporation and washing away the polymer after the golden mesh had been deposited on a substrate.
Goda’s team studied the fiber’s optical properties and determined that the best dimensions for the gold mesh were a wire diameter of 490 nm with a deposited gold thickness of 150 nm. Optimizing the nanomesh structure to get the best SERS performance without compromising other desirable qualities, such as stretchability and flexibility, was the most challenging part of the research, Goda says.
Proof of concept
To test their wearable gold mesh patch, the researchers performed SERS on the device in the presence of solutions containing rhodamine 6G, a fluorescent dye, in varying concentrations. With laser light at 785 nm and a portable SERS detector, the experiment picked up the dye down to a molar concentration of only 10 × 10–9.
“The sensitivity of our nanomesh sensor is comparable with that of other types of wearable sensors,” Goda says. “As the conventional wearable sensors are usually tailored for the target analyte based on the prior knowledge of the analyte, their sensitivity can be very high while they sacrifice the capability of multiplexed chemical sensing and identification of unknown analytes.”
Although very thin, the gold nanomesh sensor is extremely durable and can be stretched and deformed without breaking. Therefore, it can be adhered to many different surfaces—not just human skin—for different sensing purposes. [Image: ©2022 Goda et al.] [Enlarge image]
The researchers put the nanomesh patch through 1,000 mechanical crumpling cycles and 1,000 stretching cycles at 50% strain, without detectable damage to SERS sensitivity. The team also stuck mesh samples to human skin, a face mask, an elevator control panel, a computer keyboard and other surfaces to detect sweat biomarkers (urea and ascorbic acid) as well as drugs, such as methamphetamine and cocaine. They even put the patch in water to test for microparticles, such as polyethylene, to demonstrate that the nanomesh could detect ocean pollution.
Further steps
Although the researchers’ work involved an external laser and SERS detector, Goda says combining the components with the mesh would be possible in the future. One way to do it would be to integrate a nanolaser and nanospectrometer with the nanomesh on a chip.
The team will continue to improve the sensitivity and specificity of the wearable gold-mesh sensor. “With these improvements,” Goda says, “we want to explore more applications, such as infectious disease sensing for disease control and prevention, urine drug testing for law enforcement, pesticide residue detection for food safety, microplastic detection for inspecting drinking water and oceans, blood glucose detection for monitoring diabetes and toxic material detection for environmental safety.”