![]()
Francesca Calegari [Image: DESY, M. Mayer]
This year’s Conference on Lasers and Electro-Optics Europe and European Quantum Electronics Conference (CLEO/Europe-EQEC) will be held in Munich, Germany, from 26 to 30 June 2023. One of the keynote speakers for CLEO/Europe is Francesca Calegari, who is a physics professor at the Universität Hamburg, Germany, and leads the Attosecond Science division at the Center for Free-Electron Laser Science at Deutsches Elektronen-Synchrotron (DESY). OPN chatted with Calegari prior to the event to get a preview of her upcoming talk entitled “Ultrafast attosecond and few-fs sources for control of molecular reactivity at the electron timescale.”
What is the motivation behind studying molecular reactivity?
Francesca Calegari: The idea is to access molecular dynamics at extreme timescales, where one can act on the electron dynamics. What has been done in the past initiated femtochemistry, where the use of femtosecond laser pulses controls the chemical reactivity of molecules. This was done by acting on the structural dynamics—so the nuclear dynamics—at their own timescales.
Our approach is different in that, thanks to the use of extremely short light pulses, one can access the electron dynamics. Here, the major goal is to act directly on the electrons’ positions or the distributions of the electrons within a molecule, which are important for bond formation and breaking. So we can really directly act on the reactivity when we play with the electron density distribution in the molecule.
Of course, chemical reactivity is behind many processes. In fact, it is the chemical reactivity that allows molecules to serve different functionalities, like biological functionalities such as light harvesting. So the primary goal is to understand whether we can control the chemical reactivity by acting on the electron dynamics, and then to affect the final functionality of the molecule.
Can you talk about that need for ultrashort light sources?
So we need attosecond and few-femtosecond light pulses to study what we want to study. Attosecond light pulses are mostly produced in the extreme ultraviolet or even X-ray range. That’s because, intrinsically, the pulse-generation process starts from the infrared laser light and produces photons at much higher photon energies, ending up with photons that are extreme ultraviolet, which is known to be an ionizing radiation.
“ We have been searching and pushing for the combination of the attosecond and few-femtosecond light pulses produced in the ultraviolet ”
With this, you can study what happens to molecules when you ionize them. For instance, we have been studying DNA building blocks such as adenine, looking into their ultrafast responses to ionization and how we could control the dissociation of the molecule by acting at extreme timescales. But of course, this is not something that typically happens on Earth because ionizing radiation is not penetrating our atmosphere.
That’s why we have been searching and pushing for the combination of the attosecond and few-femtosecond light pulses produced in the ultraviolet. This is a very interesting photon energy range. At least a portion of the ultraviolet light does in fact penetrate our atmosphere, and it activates a number of photochemical and photobiological processes. But the challenge here is that ultraviolet radiation is difficult to produce at very short pulse durations. So we are really trying tackle the problem of generating ultrashort ultraviolet pulses and combining them with our attosecond sources for time-resolved measurements.
How are those sources being developed?
We design and create table-top light sources that are driven by an ultrashort laser. So we typically start from a commercial laser source that operates in the femtosecond domain, and then we use nonlinear optical processes to shift the wavelength and get shorter and shorter path durations. For generating attosecond pulses, we use high-order harmonic generation, which is an extremely nonlinear process. For this, we focus our infrared laser pulses at very high intensities on a gas target, which causes electrons to ionize, accelerate and recombine. And in this recombination with the gas target, they emit photons at high energies.
We are now optimizing this process in the ultraviolet and in the soft X-ray spectral range, which covers the so-called “water window.” There, the radiation is not absorbed by water, but by the atoms typically found in organic molecules—for instance, carbon. So you can identify signatures of absorption from such molecules in water.
For the UV-light generation, we use the process of third-harmonic generation of our infrared laser pulses. And we are now developing special devices for confining gases at high pressure over short distances, trying to make this third-harmonic generation more efficient. This will lead to producing short UV-light pulses with sufficient energy for our experiments.
Could you give an example of an experimental application of ultrashort pulses?
Previously, we showed that, indeed, we can control the molecular properties by using these ultrashort UV-light pulses. In 2019, we published a paper that reported the world record for the shortest UV-light pulses ever generated. These are sub-two-femtosecond UV-light pulses. And this was just a demonstration to show that we can produce and characterize such short UV-light pulses.
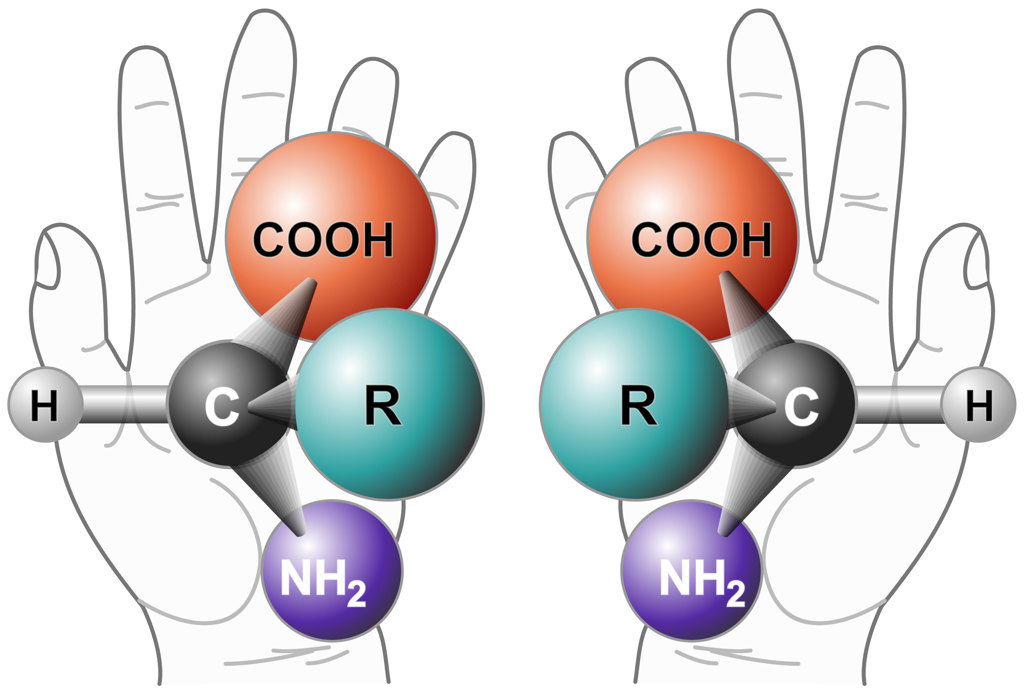
Two enantiomers of a generic amino acid. [Image: NASA / Wikimedia Commons]
Recently, we have been using those light pulses to study the excitation of chiral molecules. Chiral molecules are a special class of molecules that have the same chemical compositions but are mirror images of one another. So you have two enantiomers that have completely different chemical properties even though their compositions are the same. Chirality is a very important subject in life sciences because living organisms are composed mostly of one enantiomer, and for pharmaceutical applications, because sometimes one enantiomer is used for healing while its mirror image is highly toxic. So it’s very important to recognize which enantiomer we are dealing with.
What was your aim in exciting the chiral molecules?
What we have been investigating is the enantiomers’ response to light. In our experiment, we were initiating electron dynamics in methyl lactate, which is the chiral molecule important for muscle metabolism. Our very short UV pulses can excite a number of electronic states, which is a prerequisite for initiating these electron dynamics such that electrons are moving very rapidly into the molecule.
So we wanted to probe the chiral response of the molecule as a function of the electron dynamics. We used circularly polarized light, which is also chiral. When a chiral molecule interacts with circularly polarized light, it shows different responses depending on which enantiomer it is. So you use this circularly polarized light pulse to ionize the molecule, and depending on which enantiomer it is, the emission would be forward or backward. You get an asymmetric photoemission, which is due to the chiral response of the molecule. This method is called photoelectron circular dichroism. You can read further in our arXiv paper.
Update, 3 April 2023, 12:50 EDT: This story has been updated to replace a discussion of several details of recent work with a link to the posted preprint on arXiv.
