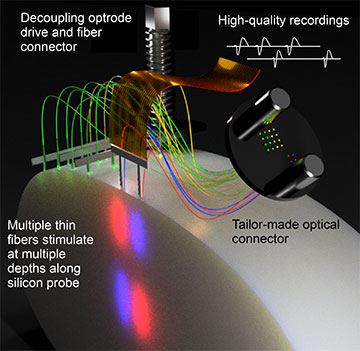
Scheme of the new Fused Fiber Light Emission and eXtracellular Recording (FFLEXR). [Image: Research group Ilka Diester / University of Freiburg] [Enlarge image]
The advent of optogenetics has ushered in a new era of neuroscience, as it allows researchers to activate or inhibit specific neurons with light in freely moving animals. Combined with electrophysiological recordings, optogenetic tools can unravel the complex interactions between neurons during everyday functions as well as within disease states.
The optical fibers for optogenetics typically have a cross-section of several orders of magnitude larger than a wire electrode or brain cell, which can damage the recorded neurons and interfere with experimental results. Now, researchers in Germany have developed an improved setup based on ultrathin optical fibers that cause minimal disturbance to surrounding brain tissue (Nat. Commun., doi: 10.1038/s41467-022-28629-6).
Cell-sized optical fibers
A common approach to combining optogenetic manipulation and electrophysiological recordings is to use a large-diameter optical fiber that illuminates the delicate tissue surrounding the electrode. However, such bulky fibers not only limit the number of channels on an animal’s head, but they can negatively affect recording quality due to photochemical and electromagnetic interference, photovoltaic effects and mechanical damage when implanted.
“Simultaneous large-scale recordings and optogenetic interventions may hold the key to deciphering the fast-paced and multifaceted dialogue between neurons that sustains brain function,” said first author David Eriksson from the Optophysiology Laboratory at the University of Freiburg, Germany. “Whereas electrodes have experienced a huge development from single wires to hundreds of recording channels and decreasing cross-sections, the optical fibers have not been refined in terms of size and number of stimulation channels.”
To mitigate these issues, Eriksson and his colleagues decided to replace a single thick optical fiber with multiple ultrathin, cell-sized optical fibers that surround the electrode. In the process, they had to develop a completely new optical framework—which they termed Fused Fiber Light Emission and eXtracellular Recording (FFLEXR)—that still allowed recordings and stimulations to be done in a freely moving animal.
Successful neuronal stimulation
FFLEXR employs ultrathin fibers with outer and core diameters of 30 and 24 microns, respectively, that can be attached to any silicon probe for implantation. Unlike traditional thick fibers with a core diameter of 200 microns, FFLEXR’s fibers are very flexible with a much smaller bending radius, allowing the animals to move unencumbered. The experimental setup also includes a lightweight fiber-matrix connector, an optical commutator for efficient multichannel stimulation, and a general-purpose patch cable.
“Our alternative approach maintains the flexibility to apply any desired wavelength via an external interchangeable light source and enable optogenetic stimulation at different depths of brain tissue,” said senior author Ilka Diester, also from the Optophysiology Laboratory at the University of Freiburg.
The researchers validated their system by conducting simultaneous optogenetic manipulation, electrophysiological recordings, and behavioral readout in freely moving mice and rats. In both cases, the animals were implanted with multiple minimally invasive ultrathin optical fibers as well as laminar probes for high-quality extracellular recordings. Eventually, they aim to expand FFLEXR to achieve whole-brain surface recording and optogenetic stimulations.
“A future usage of this system could be to record neuronal activity from those fibers,” said Eriksson. “This could be a way to study spatially resolved neuromodulatory signals, which in turn are thought to be crucial for understanding multiple types of diseases.”