[Image: Morsa Images/Getty Images]
The world is understandably preoccupied with COVID-19 vaccines and the prospects they offer for emerging from the pandemic’s long shadow. But the vaccine rollout has been far from uniform from country to country. And if the experience with SARS-CoV-2, the coronavirus behind the pandemic, has taught the world anything, it’s the importance of being able to diagnose infections fast, early and often—to track the spread of new viruses and to prevent local outbreaks from becoming global scourges.
Thus research into more rapid, effective tests for SARS-CoV-2 and other viruses continues apace. Indeed, the past several weeks have seen a flurry of research reports on approaches to improve existing testing methods and on other, alternative modes for COVID diagnostics. And many of them rely at least partly on optical and photonic technologies, ranging from fluorescence to plasmonics to holography and deep learning.
Improving the “gold standard”
A number of recent findings have focused on ways to speed up or otherwise improve what is commonly referred to as the “gold standard” of COVID testing, reverse-transcriptase polymerase chain reaction (RT-PCR). This method involves identifying traces of a virus’ genetic signature in a sample from a patient, by first transcribing fragments of viral RNA to DNA (the RT part) and then vastly amplifying the DNA to reach detectable levels (the PCR part).
[Image: Getty Images]
In one key approach, real-time or quantitative PCR (qPCR), a probe attached to a fluorescent molecule is added to the mix; with each round of amplification, fluorophores are released into the buffer solution, allowing the presence of the virus, and the viral load, to be detected optically after amplification. (For more on the technique, see “Optics and the COVID-19 Pandemic,” OPN, May 2020.)
While RT-PCR is considered the best current test for COVID-19, it has its drawbacks. One is that sufficiently amplifying the viral RNA takes 30 to 40 rounds of thermal cycling—heating and cooling—in bulky, specialized machines, necessitating an hour or more to complete the test, and generally requiring that samples be sent away to third-party labs. Thus patients may need to wait for several days to get a result. Further, as RT-PCR testing has ramped up in the face of the pandemic, some parts of the world have reportedly grappled with shortages of the chemical reagents needed to drive the technique.
A dash of plasmonics
Recent research led by scientists at the Korean Advanced Institute of Science and Technology (KAIST), Republic of Korea, has taken aim at some of these defects of RT-PCR by throwing in a dash of plasmonics (ACS Nano, doi: 10.1021/acsnano.1c02154).

Researchers in the Republic of Korea report that they have developed a plasmonic–microfluidic chip that can amplify DNA much faster than conventional, benchtop PCR, and that thus might speed turnaround in COVID-19 testing. [Image: Adapted from ACS Nano 2021, DOI: 10.1021/acsnano.1c02154]
The KAIST team has developed a prototype microfluidic chip in which tiny samples from patients are sucked into the chip and shunted to an array of microscopic reaction chambers. The tiny chambers are underlain by row upon row of still smaller glass “nanopillars,” each topped by a “nanoisland” of gold. When a white LED illuminates the sample, these subwavelength gold islands plasmonically confine and enhance the optical field, rapidly converting the light to highly localized heat. Turning off the LED allows similarly rapid cooling.
This rapid, localized plasmonic heating and cooling, the researchers report, allows the plasmofluidic chip—which is approximately 14×22 mm in area—to accomplish the required 40 thermal cycles for PCR in as little as five minutes. This, the team maintains, allows the prototype to complete a full-fledged RT-PCR test for COVID-19, from sample loading to fluorescent readout, in as little as 10 to 13 minutes, versus an hour or more for conventional methods. The group concludes that the platform “can provide many opportunities for rapid [point of care] molecular diagnostics in slowing the fast-spreading pandemic.”
Conserving scarce reagents
Microfluidics also offers an advantage in another PCR quandary: conserving the reagents required for the technique. In many areas, these reagents have been hit by supply-chain squeezes in the face of the huge demand for COVID-19 testing.
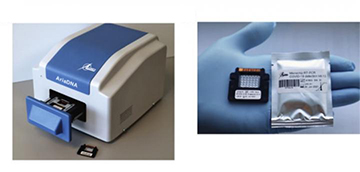
The Lumex Instruments AriaDNA unit (left) and a microfluidic array used in COVID-19 testing (right). Researchers report that the use of microfluidic technology reudces both the use of scarce RT-PCR reagents and the time required for testing. [Image: Lumex Instruments Canada]
Researchers at Simon Fraser University, St. Paul’s Hospital and the University of British Columbia, Canada, in partnership with the Canadian firm Lumex Instruments, have validated a microfluidic-based technique that reportedly is quite stingy with these scarce reagents (J. Mol. Diagn., doi: 10.1016/j.jmoldx.2021.02.009). The team’s work suggests that the system—a “user friendly,” microchip-based and compact setup that performs PCR tests on the nasopharyngeal swabs commonly used to obtain patient samples—requires reagent amounts ten times lower than in a conventional COVID-19 PCR test.
The team also found that its lightweight setup, which draws only 100 watts of power, could complete a test for the SARS-CoV-2 coronavirus in only 30 minutes. And the team’s tests agreed 100% with results from conventional clinical techniques.
From PCR to LAMP
The disadvantages of “gold-standard” RT-PCR—especially the dozens of rounds of thermal cycling required—have encouraged researchers to look at other systems of gene amplification that might be pressed into service for COVID-19 testing. One of those is reverse-transcriptase loop-mediated isothermal amplification (RT-LAMP), which has been shown to detect infection by the coronavirus.
RT-LAMP tweaks the chemistry of gene amplification by using multiple sets of nucleic-acid primers that amplify multiple regions on the target gene. This increases the method’s specificity relative to PCR. One result is that, instead of requiring 30 to 40 rounds of thermal cycling, RT-LAMP can be carried out at a single temperature. And, by putting fluorescent dyes and laser or LED excitation into the mix, the viral presence can be scoped out with the naked eye by a simple color change.

The handheld, battery-powered testing device prototyped by researchers at the University of Illinois, Urbana-Champaign, USA, reportedly can provide accurate COVID-19 test results in half an hour, using the RT-LAMP gene-amplification technique. [Image: Photo by L. Brian Stauffer]
A recent project at the University of Illinois, Urbana-Champaign, USA, found a way to wrap an RT-LAMP system for SARS-CoV-2 detection into a 3D-printable, battery-powered, handheld device—one that reportedly can detect the presence of the coronavirus at concentrations of as little as one viral particle per microliter of sample (Nat. Commun., doi: 10.1038/s41467-021-23185-x). Tests with the device—dubbed Scalable and Portable Testing (SPOT)—on 104 clinical saliva samples showed that it accurately identified 28 out of 30 SARS-CoV-2-positive samples (93.3% sensitivity, or true-positive rate) and 73 of 74 SARS-CoV-2-negative samples (98.6% specificity, or true-negative rate).
While the SPOT system is still in prototype, the team believes that the final device might cost less than US$78. The system’s “combination of speed, accuracy, sensitivity and portability,” the authors conclude, “will enable high-volume, low-cost access to areas in need of urgent COVID-19 testing capabilities.”
Beyond gene amplification
While methods based on gene amplification constitute the most common way to sniff out the presence of a coronavirus infection, other approaches are possible. A variety of teams are looking at techniques that zero in on not on the presence of viral RNA, but on specific biomarkers or antibodies that signal the presence of the disease.
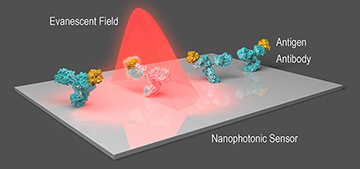
A research team led by OSA Fellow Laura Lechuga, Catalan Institute of Nanoscience and Nanotechnology, Spain, for example, is exploring the development of nanophotonic biosensors that could be used in COVID-19 diagnostics and surveillance. The work is proceeding through CONVAT, a project funded under the EU’s Horizon 2020 framework funding program. Lechuga’s team reported on progress in a paper earlier this year (J. Phys. Photonics, doi: 10.1088/2515-7647/abd4ee).
The nanophotonic sensor being developed by the team of Laura Lechuga, Catalan Institute of Nanoscience and Nanotechnology, Spain, senses changes in local refractive index as virus particles glom onto antibodies affixed to the sensor. [Image: OPN, April 2020] [Enlarge image]
The project revolves around the creation of an integrated biosensor that uses a bimodal-waveguide-based interferometer that’s primed with COVID-19 antibodies. These antibodies fit, lock-and-key style, with antigens on the virus, and thus cause viral particles in the sample to clump along the waveguide. The presence of the virus changes the local refractive index, which is in turn read in changes in the interference pattern of evanescent waves from the bimodal waveguide. It’s an approach that the lab has already demonstrated for detecting other clinical biomarkers (see “Nanophotonic Biosensors,” OPN, April 2020).
Infrared and TR-FRET detection
Other groups have recently put their own spin on direct detection of viral particles, biomarkers or antibodies. Researchers from Monash University and the Peter Doherty Institute for Infection and Immunity, Australia, for instance, have reported a proof-of-concept of a simple, infrared-based approach that screens for COVID-19 biomarkers in saliva samples (Angew. Chem. Intl. Ed., doi: 10.1002/anie.202104453).
In this approach, a patient with COVID-19 symptoms “dribbles out” a stream of saliva onto an infrared transflection substrate. The sample is dried, and its infrared spectra are read using an off-the-shelf portable infrared spectrometer (modded to allow for contactless, high-throughput scanning). The spectra—which, according to the authors, constitute “a chemical snapshot of the entire saliva chemistry, including COVID-19 markers”—then pass to a computer for statistical analysis, to infer the likelihood of a coronavirus infection. The team says that, using this approach, it was able to identify the signature of SARS-CoV-2 in 27 of 29 infected patients that it tested.
[Image: Kateryna Kon/Science Photo Library/Getty Images]
In yet another take on detection of SARS-CoV-2, researchers affiliated with the University of Helsinki, Finland, say they have developed an antigen test for the virus that can be conducted in as little as 10 minutes (mBio, doi: 10.1128/mBio.00902-21). The approach uses the optical technique known as time-resolved Förster resonance energy transfer (TR-FRET).
TR-FRET works because pairs of different fluorophores—called donors and acceptors—can transfer energy to one another when they’re in close enough proximity, an exchange that leads to the emission of a photon at a distinct wavelength. In the Finnish team’s system, SARS-CoV-2 antibodies are labeled with such donor and acceptor fluorphores and put into the sample to be tested. The presence of virus in the sample will cause the antibodies to bind simultaneously against the virus, bringing the fluorophores into closer contact and causing measurable emissions of light.
The team reportedly found high specificity and sensitivity in the tests it conducted. And the researchers believe that “the test format could easily be adapted to high-throughput testing” and could “be applicable to a wide variety of infectious and perhaps also noninfectious diseases.”
RBCs, holography and deep learning
Finally, an intriguing and very different approach to coronavirus detection was proposed in early May by a research group headed by OSA Fellow Bahram Javidi, University of Connecticut, USA (Opt. Lett., doi: 10.1364/OL.426152). This approach hinges not on detecting viral RNA or antibodies, but on picking up the impact of COVID-19 on a patient’s red blood cells (RBCs).
Specifically, previous work from a variety of labs has shown that, particularly in severe cases, COVID-19 can cause measurable changes in laboratory parameters such as the RBC distribution width (an indicator of RBC volume and size variation), as well as other aspects of RBC morphology. Javidi’s group suggests that these changes might be detected in blood smears from patients in a compact, field-ready setup, through a combination of digital holographic microscopy (DHM) and deep learning.
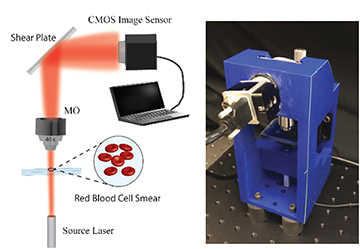
Left: The optical setup of a digital holographic microsocopy approach for detecting COVID-19 via its effects on red blood cells, tested by the team of Bahram Javidi, University of Connecticut, USA. Right: The 3D-printed prototype used in the experiments. [Image: T. O’Connor et al., Opt. Lett. 46, 2344 (2021)] [Enlarge image]
In the team’s 3D-printable prototype device, an RBC smear from a patient—such as one might get from a finger prick in a doctor’s office—is placed on a microscope slide in a digital-holography microscope setup. A 635-nm diode laser beam passes through the sample and is magnified through a microscope objective, after which the transmitted beam strikes an angled glass plate. Reflections from the front and back surfaces of the glass create two reflected beams, separated by a lateral shear, which then self-interfere. The resulting time-varying interference patterns—digital holograms—are captured, in 10-second, 20-fps videos, by a CCD camera.
Next, computer Fourier analysis extracts the phase profiles of the RBCs from the video hologram data. Features pulled from the phase profiles are then piped into a deep-learning recurrent neural network (RNN) that’s been trained to classify samples as COVID-positive or COVID-negative based on RBC morphology. In preliminary tests with 24 separate blood samples, the team found that the setup correctly identified eight of ten patients known to be COVID-positive (80% sensitivity), and also correctly identified 13 of 14 individuals known to be COVID-negative (92.86% specificity).
While the researchers see some distinct advantages to their DHM system in “cost, accessibility and time to results,” they acknowledge that it does have some drawbacks. In particular, it’s unclear whether the RBC morphology effects found in moderate and severe COVID cases, such as those studied in the proof-of-concept, can also be used for detection in asymptomatic or mild cases. The team plans to continue working on validating the system’s effectiveness in detecting milder cases, and on exploring the use of “different cell types such as white blood cells for COVID classification.”
Optics’ continued role
Like many researchers working to find better, faster COVID-19 tests, Javidi hopes that the work may particularly benefit areas that have struggled with resources to fight the pandemic. “Countries with under-resourced health care systems may benefit from this low-cost, rapid testing,” he told OPN.
And the bevy of coronavirus testing improvements now being trialed also points to the continued role of optical techniques, such as the holography at the center of the work of Javidi’s team, in addressing this and other global problems.
“This year is the 50th anniversary of [Dennis] Gabor’s Nobel Prize for holography,” noted Javidi. “It is great to see that holography continues to be applied in new and important ways.”