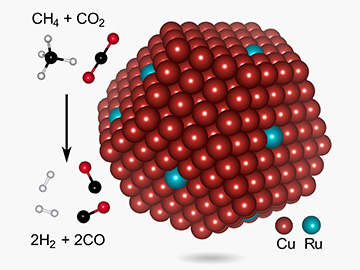
A research team led by scientists at Rice University’s Laboratory of Nanophotonics created a copper–ruthenium nanoparticle photocatalyst that can the researchers report dramatically cut the energy input for syngas production. The team boosted the photocatalyst’s stability by shrinking the active sites to single atoms of ruthenium (blue). [Image: John Mark Martirez/UCLA]
Greener chemical manufacturing might soon be available at the switch of a light. That’s the goal of researchers led by a team at Rice University’s Laboratory of Nanophotonics (LANP), who report a photocatalyst comprising a copper nanoparticle studded with isolated single atoms of ruthenium to make syngas, a vital chemical feedstock (Nat. Energy, doi: 10.1038/s41560-019-0517-9).
Because the new nanoparticle catalyst is activated by light from an optical-fiber laser source, says LANP director and OSA Fellow Naomi Halas, it could drastically reduce the energy input required to make syngas, as well as inspire design of other photocatalysts to improve the chemical industry’s enormous carbon footprint. The chemical industry uses on the order of 25%– 30% of the total U.S. manufacturing energy consumption annually, according to Halas.
Rice postdoctoral research fellow Linan Zhou is the lead author of the paper, with additional co-authors from Rice, the University of California (UC), Santa Barbara; UC Los Angeles, and Princeton University, USA.
Getting away from the heat
Syngas—which consists mainly of carbon monoxide and hydrogen—can be generated through a process known as methane dry reforming (MDR). In this process, methane reacts with water or carbon dioxide to derive the feedstock end-product. While MDR has been considered more environmentally sound because the recipe combines two potent greenhouse gases, the reaction requires temperatures on order of 800 °F (some 425 °C).

Naomi Halas. [Image: Jeff Fitlow/Rice University]
Indeed, the chemical industry for the last century has relied almost exclusively on heat and pressure to make its products, Halas says. What’s more, the catalysts used in manufacturing syngas and other products are prone to a deactivating, carbon-waste buildup known as coking, an inefficiency industry would be happy to eliminate.
With the new light-activated copper-ruthenium catalyst, the reaction temperature to make syngas can be lowered to about 200 °F, a huge difference, Halas says. In fact, “you could do it in your oven at home with the right light source.”
Save the catalysts
As for catalyst depletion, Halas says, the new photocatalyst was tested for 50 hours with “absolutely no depletion.” While the researchers used an optical-fiber laser source, she adds that, with sufficient intensity, “it’s a great application for solid-state lighting—LEDs.”
The copper–ruthenium photocatalyst builds on Halas’ work with plasmonic metal nanostructures, in which light interacts strongly with a metal to create a “hot antenna” that delivers the energy required to initiate and sustain chemical catalysis.
Ruthenium itself doesn’t absorb light, Halas explains, and so the photocatalytic process for making syngas depends on the copper nanoparticle, which acts as just such an antenna to deliver the energy required to the individual ruthenium atoms that dot the nanoparticle. Quantum-mechanical modeling suggests that the single-atom approach reduces the barrier to chemical reaction and thus boosts efficiency.
Cheaper hydrogen, on demand

Rice University postdoc Linan Zhou is the lead author on the new study. [Image: Jeff Fitlow/Rice University]
The photocatalyst could also be used to make hydrogen on demand, Halas says, a big plus for refueling hydrogen fuel cells such as those used to power motor vehicles. “When you turn on the light, you make hydrogen.”
“Hydrogen is part of our future,” Halas said in a video interview at CLEO 2019, where she was a plenary speaker. However, “right now, the cost of hydrogen is 10 times that of gasoline, so it’s not commercially effective.” With a light-based catalyst, according to Halas, the energy cost of manufacturing hydrogen could be reduced, and it could be made in compact equipment.
And for chemists, a photocatalyst provides unparalleled control of chemical reactions, Halas says. “Not only do you save huge amounts of energy, but like nothing else can you control [reactions] with the on-off switch of a light. We’re doing chemistry in a whole new way.”
Light and commerce
The light-activated catalyst technology could soon become commercially available to the chemical industry, Halas says. The technology has been shown to work at scale, she says, and is under license by Syzgy Plasmonics, a startup whose co-founders include Halas and Rice University colleague and co-author Peter Nordlander.
Through commercial licensing, Halas says, any needed improvements to the catalyst would emerge and could be fine-tuned by engineers. Making syngas with the new photocatalyst would require a new type of reaction chamber, however. “You have to have windows to let in the light,” Halas says.
