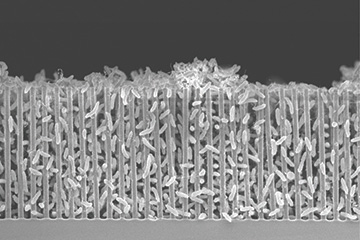
Scanning electron micrograph of a nanowire–bacteria hybrid array. Work by a U.S.-based team has optimized the array as an artificial-photosynthesis chemical factory for creating acetate. Area imaged is approximately 50 microns across. [Image: Peidong Yang/UC Berkeley]
A research team led by scientists at the University of California, Berkeley, USA, has reported an optimized approach to artificial photosynthesis that marries biology and hardware to create a miniature, light-driven chemical factory (Joule, doi: 10.1016/j.joule.2020.03.001). The team’s device uses an array of silicon nanowires to deliver solar energy to bacteria—prompting the bugs to use atmospheric CO2 to produce versatile organic compounds like acetate. The acetate, the team says, could in turn be used to make other products like plastics, fuels, pharmaceuticals and more.
The work’s lead author, Berkeley chemist Peidong Yang, believes that the system could not only help to relieve carbon pollution on Earth, but could also produce chemical building blocks for astronauts to make critical supplies and equipment for survival on deep-space missions, including to Mars.
A hybrid approach to fixing carbon
Yang has pioneered study of nanowires as photovoltaic devices. “Our artificial-photosynthesis work started more than 15 years ago,” he explains. “We took two different approaches—one based purely on semiconductor/inorganic catalysts, and the other based on this abiotic/biotic interface, which we started around 2010,” he says.
For the current work, Yang says that the nature of the net reaction—CO2 + water + sunlight = chemicals/fuels + oxygen—makes it “quite natural for us to think about CO2 mitigation on Earth, as well as chemicals/fuels production in space.” He notes that Mars’ atmosphere comprises primarily CO2 and that frozen water exists there in abundance as well.
“Biohybrid CO2-fixing systems, particularly those driven by renewable solar energy, represent a promising strategy for sustainable CO2-to-chemical conversion,” the authors write in their paper. But the microorganism–semiconductor cathode interface had never been systematically studied with an eye toward boosting solar conversion efficiency, they note.
Feeding the bugs
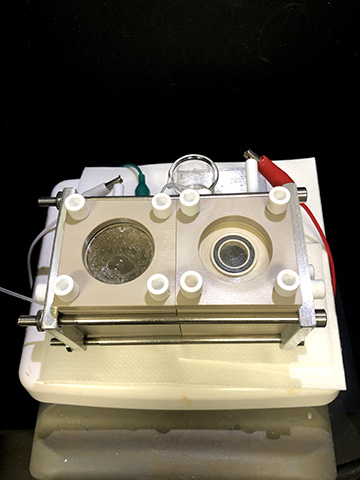
In the team’s device, the chamber on the left contains the nanowire–bacteria hybrid that reduces carbon dioxide to form acetate. Oxygen is produced in the chamber on the right. [Image: Peidong Yang/UC Berkeley]
To do so, the research team built an experimental device to house a photoactive Si nanowire array that, when packed with the bacterium Sporomusa ovata, allowed for unassisted solar-to-chemical production of acetate. The device relied on an external solar panel to feed electrons to the bacteria via the nanowires. But alternatives include using the nanowires themselves directly as solar panels to collect and transfer energy to the bacteria, or embedding quantum dots in the bacteria to absorb light energy to drive the chemical process without the need for wires.
Previous work with the nanowire array and bacteria setup, Yang says, indicated a solar conversion efficiency of only about 0.4%—far lower than the conversion rate for electrochemical systems composed of inorganic catalysts. One method Yang tried previously to boost efficiency was to pack the array with more bacteria. But instead the bugs swam away from the nanowire forest, breaking the reactor circuit.
The current study focused on the cathode nanowire/microorganism interface. What the researchers found, through a trial-and-error approach, was that acetate production by the bacteria was fouling the system, making it too alkaline for the bacteria. Not liking the environs, they would not pack densely enough in the nanowire array to move the needle on CO2 conversion efficiency.
Tuning pH, boosting efficiency
Tuning the pH of the starting electrolyte medium was the answer. The researchers began their experiments with a pH of 7.2, then 6.7, and finally 6.4. At that lowest pH, they were able to boost solar conversion efficiency to 3.6% of sustained acetate production for seven days.
The work “makes clear that the local chemical environment is evolving—pH is changing—in a way that would impact on the nanowire interface and therefore disrupt the CO2 fixation process,” Yang says. It also gives insights that will allow researchers to explore using different bacteria powered by solar energy to fix CO2 or to make different products, he says.
Interestingly, at optimized pH, the bacteria became densely packed in the nanowire array, Yang says. This fits with earlier research indicating there is “some favorable electrostatic interaction between the nanowires and these bugs,” he says.
Yang says it’s possible that the technology one day could be commercialized and used, perhaps, for larger-scale chemical manufacturing but that would depend on many variables, including device costs and performance, market opportunity, availability of venture capital, and more.
For now, “S. ovata is our model bug to test our design concept,” Yang says. “One can certainly use other bugs for different chemical products and maybe with even better yield,” he says. “In this work, we have solved the loading-density issue. One can further select or even [genetically] engineer certain bugs with even better efficiency.”
