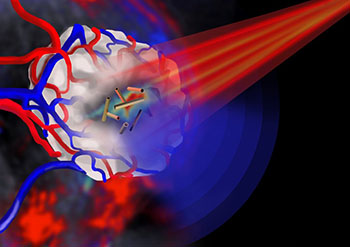
Pulsed near-infrared light (red beam), shone onto a tumor with embedded gold nanotubes as contrast agents, allows the tumor to be imaged via multispectral optoacoustic tomography (blue waves). [Image: Jing Claussen, Ithera Medical, Germany]
Scientists at the University of Leeds and the University of Cambridge, U.K., have developed a way to build uniform, hollow gold nanotubes that, combined with near-infrared (NIR) light, can serve as a sort of triple threat to cancer cells—as an imaging system, a drug-delivery system, and an army of tiny heat guns to potentially burn away tumors (Adv. Func. Mater., doi: 10.1002/adfm.201404358). A member of the research team, James McLaughlan of Leeds, described the work as “the first demonstration of the production, and use for imaging and cancer therapy, of gold nanotubes that strongly absorb light within the ‘optical window’ of biological tissue.”
The promise of gold nanotubes in biomedicine has been discussed for some time, but progress has stalled owing to the difficulty in controlling the length and uniformity of the structures, and in tuning them for optical absorption in NIR wavelengths. There’s also been concern about potential toxicity of gold nanoparticles generally.
To address these points, the Leeds-Cambridge team developed new fabrication method. The process starts with growing nanorods of silver, the length of which can be controlled by tweaking the chemistry and temperature of the growth medium. Then, in a second step, a galvanic replacement process substitutes gold for surface silver.
The result of the process is open-ended, hollow gold nanotubes of tightly controllable lengths, over a range of 300 to 700 nm—a dimension that gives the structures a strong surface plasmon resonance in the NIR region of the spectrum, with the specifics tunable by adjusting the nanotube length. As a final touch, the tubes are coated in a polymer, poly(sodium 4-styrenesulfonate), that helps stabilize the tubes and reduces their potential toxicity to cells.
In in vitro studies, the team established that the new nanotubes were absorbed by colorectal cancer cells and macrophages and, when subjected to pulsed NIR laser light, generated sufficient heat to destroy cancer cells through photothermal ablation. When injected in vivo into tumor-bearing mice, the nanotubes accumulated into the cancer cells, where they served ably as contrast agents to enhance photoacoustic imaging of the tumor. Further studies suggested that the nanotubes were cleared from the mice within 72 hours, a good sign for limiting toxicity. And the size of the hollow tubes potentially enables them to be packed with a range of potential drug payloads, from small molecules to proteins.
Taken together, according to the study’s lead author, Sunjie Ye, this combination suggests that these nanostructures—which, he says, resemble “tiny drinking straws”—could enhance conventional approaches to therapy by “integrating diagnosis and therapy into a single system.” The team is now working to move the technology toward early clinical trials.
