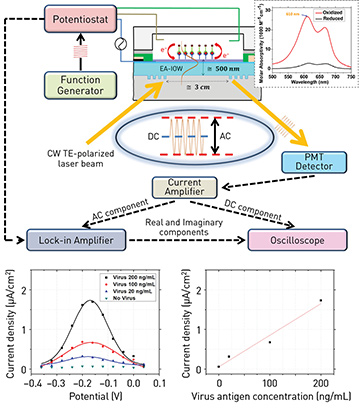
 Top: Multilayer, single-mode, electro-active, integrated optical waveguide device, functionalized with a sandwich bioassay for influenza virus detection. Inset: spectral molar absorptivity of methylene blue conjugated to the secondary antibody at different oxidation states. Bottom left: Optically reconstructed electrical-current signal from redox probe under AC potential modulation at varying bias potential. Bottom right: Virus detection at different sample concentrations.
Top: Multilayer, single-mode, electro-active, integrated optical waveguide device, functionalized with a sandwich bioassay for influenza virus detection. Inset: spectral molar absorptivity of methylene blue conjugated to the secondary antibody at different oxidation states. Bottom left: Optically reconstructed electrical-current signal from redox probe under AC potential modulation at varying bias potential. Bottom right: Virus detection at different sample concentrations.
Early detection and diagnosis of the different strains of influenza virus—the main cause of critical respiratory infections—are essential for treatment and for identifying pandemic outbreaks. Immunoassay-based detection, which rests on the unique binding affinity and specificity of antibody–antigen interactions, offers a potential means of virus detection, and enzyme-linked immunosorbent assays and fluorescence immunosensors have indeed been developed. Yet those methods have a number of shortcomings.
Our team has developed a strategy for immunosensor-based detection of viral pathogens that uses a sandwich bioassay on a single-mode, electro-active, integrated optical waveguide platform.1 The strategy rests on the surface functionalization of the photonic device with a capture antibody aimed at a specific virus antigen. Once the target antigen is bound to the device interface, it promotes the binding of a secondary antibody labeled with a unique optical probe. This probe—a methylene blue molecule that displays reversible changes in optical absorption throughout a reduction-oxidation transition—features an optical signal that can be electrically modulated and interrogated with high sensitivity by a propagating mode in the electro-active waveguide.
To demonstrate the platform’s capabilities for antigen detection and quantification, we targeted the hemagglutinin protein from the H5N1 avian influenza A virus. We tested virus protein solutions of varying concentrations, applying an AC voltammetric approach. We found that the optically measured peak intensity of the faradaic electric-current density associated with the redox probe was proportional to the antigen concentration, a relationship that opens a direct route for quantifying the virus analyte.
Our experimental data suggested a 3σ detection limit of
4 ng/mL for the virus antigen. This sensitivity, which surpasses several current technologies and, we believe, places our strategy at the frontiers of the state of the art, was reached through a probe that provides a biologically specific and fast response.
Equally important, the transduction mechanism itself is highly specific. It is optically locked to the probe designed for detection through use of a laser wavelength tuned to the optical transition associated with the particular redox process. It is electrochemically locked to the probe through modulation of the applied potential at the formal potential of the targeted redox events. And it is locked to surface events next to the electrode interface through confinement of the electromagnetic radiation along the optical waveguide.
The redundancy of those selective factors helps minimize unwanted false signals from interferents invariably present during detection. Further, we believe that our approach opens opportunities for unique detection and quantification of an immense variety of analytes.
Researchers
S.B. Mendes, J. Ghithan, M. Moreno, G. Sombrio, R. Chauhan and M. O’Toole, University of Louisville, Ky., USA
Reference
1. J. Ghithan et al. Opt. Lett. 42, 1205 (2017).
