![]()
A transparent glassfrog imaged with flash photography. The frog’s muscle and ventral skin transmit more than 90% of visible light and are still perfectly functional. [Image: Courtesy of Jesse Delia, Duke University]
A research team led by Carlos Taboada at Duke University, USA, used noninvasive, dye-free photoacoustic microscopy (PAM) to reveal how glassfrogs, an unusual “see-through” variety of amphibian, become more transparent as they sleep (Science, doi: 10.1126/science.abl6620). The frogs’ secret? They stash pigment-containing red blood cells (RBCs) in their reflective livers.
PAM images revealed that the frogs packed nearly 90% of their circulating RBCs into sinusoidal liver lobes while resting, and quickly released them back into their circulatory systems when active. The researchers believe that the mechanisms by which glassfrogs pull off this neat trick could also provide insights into metabolic, hemodynamic and blood-clotting research.
The right tool for the job
Many sea creatures—including species of jellyfish and squid—use transparency to hide from predators. The northern glassfrog (Hyalinobatrachium feischmanni) is one of a few vertebrates to use this type of camouflage to protect itself on land. However, unlike their invisible ocean-dwelling counterparts, glassfrogs have the added obstacle of pigmented hemoglobin-containing RBCs.
Taboada and his former postdoctoral advisor Sönke Johnsen noticed that while glassfrogs rested, they appeared to be moving the richly colored RBCs out of their blood vessels and into their livers—one of several internal organs contained in a membrane with reflective guanine crystals. Proving their liver-storage theory was challenging because the glassfrogs would quickly release their RBCs if awakened, stressed or given anesthesia.
PAM solved the researchers’ imaging problem. “PAM is the ideal tool for noninvasive imaging of red blood cells, because you don’t need to inject contrast agents, which would be very difficult for these frogs,” team member Junjie Yao said in a press release about the research. “The red blood cells themselves provide the contrast because different types of cells absorb and reflect different wavelengths of light. We could optimize our imaging systems to specifically look for red blood cells and track how much oxygen was circulating in the frog’s bodies.”
Light, sound and sleeping frogs
For their imaging demonstrations, the researchers shone biologically safe green laser light on glassfrogs sleeping upside-down (their preferred sleeping position in the wild) in clear plastic dishes. The frogs’ RBCs absorbed the green light and emitted ultrasonic waves that were recorded by an acoustic sensor. Data from the acoustic sensor were used to track the location of 80% to 90% of the RBCs to the frogs’ livers.
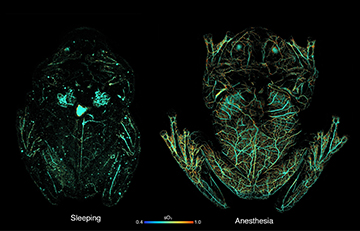
Photoacoustic-microscopy (PAM) images showing circulating red blood cells within a glassfrog while asleep (left) and under anesthesia (right). PAM uses light to induce absorption in hemoglobin, which creates sound waves; an ultrasound transducer can then map the sound waves back to the point of optical absorption. The PAM imaging shows that the frog stores red blood cells in its liver while naturally sleeping, to maintain transparency, while releasing them to the circulatory system when active or stressed. [Image: Junjie Yao, Duke University] [Enlarge image]
To confirm that the glassfrogs released RBCs from the liver into their blood vessels when active, the researchers imaged the frogs with PAM while anesthetized, which was shown in previous calibrated color photography experiments to trigger RBC recirculation while keeping the frog stationary for whole-body imaging.
The researchers also performed targeted high-resolution single-vessel/liver PAM imaging on actively moving frogs to confirm RBC flow into blood vessels and out of the liver. During these demonstrations, they found that the RBC signal in the liver increased and decreased by about 83% when the frog was resting and when the frog was active, respectively.
In addition to PAM imaging of RBCs in the liver and blood vessels, the team measured the reflectance of the guanine crystal-coated membrane covering the liver, finding that it attenuates more than 80% of incident light.
A new window into vascular medicine?
Results from the team’s study confirm that the glassfrog can regulate the location and density of RBCs in a way that improves transparency while it sleeps without causing blood clots or tissue damage. The researchers’ next steps may include figuring out why the frog’s RBCs do not clot when packed and unpacked from the liver and using that information to improve vascular and blood-clotting conditions in humans.
The team also concluded that, paired with noninvasive photoacoustic imaging, the glassfrog could prove a useful in vivo model for future physiology and biomedical-engineering research.