Artist’s representation of biochemical quantitative phase imaging with mid-infrared photothermal effect, developed by a research team at the University of Tokyo. [Image: s-graphics.co.jp, CC BY-NC-ND]
Because cells are highly transparent and do not absorb much light, light microscopy techniques often require dyes or labels to enhance image contrast. Such contrast agents, though, can disturb the normal function of living cells and even prevent their imaging.
A team of researchers from Japan has developed a hybrid, label-free microscopy technique that the researchers believe could prove well suited for studying fragile systems of living cells without damaging them (Optica, doi: 10.1364/OPTICA.390186). It combines two label-free imaging methods—quantitative phase imaging (QPI) and molecular vibrational imaging (MVI)—into a single setup that simultaneously measures the sample’s phase information and molecular components. The authors believe that the technique, called molecular vibration-sensitive QPI (MV-QPI), will aid research in areas like regenerative medicine, stem cell development, and cellular disease.
No stains, dyes or labels
The recent growth in popularity of label-free imaging reflects the desire to observe native cell behavior without introducing confounding factors. These approaches exploit the inherent contrast mechanisms of cells instead of relying on external dyes and markers.
The study’s senior author, Takuro Ideguchi, wanted to merge two complementary label-free modalities to measure different dynamic properties of a living cell at the same time. QPI gathers phase information with ptychography, or holographic microscopy, to create a map of optical-path-length shifts associated with the sample—a map that contains information about the sample’s local thickness and refractive index. MVI, on the other hand, probes the vibrational signatures of molecules with light to create chemical maps of subcellular organelles and biomolecules.
“The motivation toward this work is to develop an advanced label-free microscopy technique in terms of photodamage, sensitivity, spatial resolution and information content,” said Ideguchi, an associate professor at the University of Tokyo’s Research Institute for Photon Science and Technology. “These capabilities are needed for applications where detailed diagnosis of unperturbed biological cells is needed over an extended period of time.”
Versatile technique
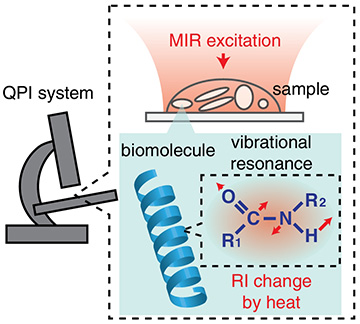
Schematic diagram of MV-QPI. [Image: M. Tamamitsu et al., Optica, doi: 10.1364/OPTICA.390186]
MV-QPI works by first illuminating a sample in the objective of a QPI microscope with mid-infrared light. This excites the resonant biomolecules to their fundamental vibrational states, and this vibrational energy is transformed into heat—a phenomenon known as the photothermal effect. The QPI system then measures the accompanying decrease of the refractive index in the vicinity of the resonant molecule.
Ideguchi and his colleagues integrated MVI capability into two distinct QPI systems to demonstrate the versatility of MV-QPI. The first setup was based on digital holography, which is a wide-field interferometric technique that provides a 2D map of phase delay. The second involved a 3D technique called optical diffraction tomography, which generates a depth-resolved map of refractive index. A mid-infrared laser for MVI and a visible laser for QPI were synchronized for both setups.
Pushing the limits
The researchers successfully validated the MV-QPI systems with various organic liquids, microbeads, and finally live cells. Future work will focus on improving the performance of the technique by increasing the imaging speed and molecular specificity with a more sensitive image sensor and a high-intensity, nanosecond mid-infrared light source.
“Our technique has the potential to push the capability of label-free microscopy by combining the benefits of the two major label-free methods,” he said. It “allows for cross-correlative analysis based on the morphological and chemical contrasts of a specimen, which could open a door to a novel and detailed characterization of a live biological cell.”
