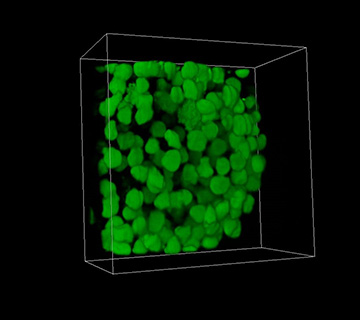
A 3-D image of a spheroid made of human embryonic kidney cells created with three-photon excitation light-sheet fluorescence microscopy. [Image: Courtesy of the University of St. Andrews]
Researchers from the University of St. Andrews, U.K., have combined three-photon excitation with light-sheet fluorescence microscopy (3P-LSFM) to increase imaging depth in biological samples with minimal cellular damage (Opt. Lett., doi: 10.1364/OL.43.005484). By combining these two modalities, they exploit light-sheet microscopy’s fast, high-resolution imaging with minimal sample damage and multiphoton excitation’s longer light wavelengths, which reduce out-of-focus light and sample damage.
Results from demonstrations comparing two-photon (2P-) with 3P-LSFM showed that the 3P setup images the surface of a sample just as well as the 2P setup, but 3P was substantially better at imaging deep within the sample. Team lead Kishan Dholakia said in a press release that the imaging approach “… could be especially useful for neuroscience and developmental biology studies and might find application in imaging multiple samples in an automated way for drug discovery.”
Improving LSFM for volumetric imaging
LSFM uses a 2-D sheet of light to image biological samples plane-by-plane. Each section of the imaged sample is only exposed to fluorescent light once. That makes the technique less phototoxic than other methods that illuminate the whole sample for the entire length of the imaging process. Still, even with comparatively low levels of light exposure, LSFM can still cause cellular damage. And LSFM does not offer a large field-of-view (FOV), which would be particularly helpful in imaging live samples.
To make LSFM more useful for imaging live biological samples, Dholakia and his team turned to multiphoton excitation. In that scheme, a fluorescently labeled molecule in the sample emits a light signal when it is excited by two or three photons from an external light source. Multiphoton excitation is a better choice for live biological samples than single-photon excitation, because it uses longer wavelengths and limited volumes of light, which are less likely to cause cellular damage and reduce out-of-focus areas.
The researchers designed demonstrations to compare 3P-LSFM imaging with 2P-LSFM. For the first demonstration, they used 1-µm-diameter blue fluorescing beads embedded in a transparent gel. The 3P-LSFM setup produced a tighter excitation confinement and less fluorescence excitation outside the FOV compared with the 2P-LSFM setup.
For the second demonstration, the researchers contrasted 3P-LSFM biological imaging with 2P-LSFM using 450-µm spheroids of human embryonic kidney cells. Both modes produced similar-quality images of the surface of the spheroids, but 3P-LSFM captured higher-contrast images from within the spheroids. The researchers quantified this performance by measuring the contrast-to-noise ratio (CNR) of images taken by both modalities at various depths. The CNR for 2P-LSFM dropped 71 percent at a depth of 450 µm, while 3P-LSFM only dropped 15 percent.
Expanding FOV with Bessel beams
The researchers also ran numerical models and simulations with fluorescent beads to see if a Bessel-beam light source, instead of the standard Gaussian beam, would improve 3P-LSFM’s depth imaging and expand the FOV. The team noted that previous research has shown that Bessel beams could improve imaging performance for 1P- and 2P-LSFM.
The numerical models and simulations showed that the Bessel-beam techniques, dubbed 2P-BB-LSFM and 3P-BB-LSFM, obtained 80-percent and 98-percent excitation confinement, respectively. 3P-BB-LSFM also produced a larger FOV than the 2P mode. Although this is positive news for 3P-BB-LSFM, the researchers acknowledge that the average power used in their Bessel-beam simulations would cause damage to sensitive biological samples. Nevertheless, the team hopes to improve its 3P-BB-LSFM technique by optimizing the laser source.
