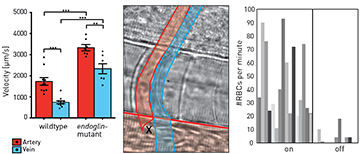
 Left: Maximum blood flow velocities in healthy fish (wild type), compared with mutant fish (endoglin-mutant) for artery (red) and vein (blue). Center: Schematic of the optical-rail scenario for restoring blood flow in capillary. Blood flows through the artery (red) at the bottom and past the optical tweezers (black cross), which redirect red blood cells into the arterial capillary (red, vertical tube) and the venous capillary (blue). Right: Number of red blood cells counted per minute with the optical rail on and off.
Left: Maximum blood flow velocities in healthy fish (wild type), compared with mutant fish (endoglin-mutant) for artery (red) and vein (blue). Center: Schematic of the optical-rail scenario for restoring blood flow in capillary. Blood flows through the artery (red) at the bottom and past the optical tweezers (black cross), which redirect red blood cells into the arterial capillary (red, vertical tube) and the venous capillary (blue). Right: Number of red blood cells counted per minute with the optical rail on and off.
Complex structures of tubular vessels (arteries, veins and capillaries) make up the cardiovascular systems in higher developed organisms. These systems enable nutrient transfer and gas and waste exchange. The correct shaping of blood vessels is vital during development and aging, and blood vessel malfunctions or diseases are among the most lethal disorders in humans. Understanding the biophysical cues underlying these ailments has become a top priority for researchers worldwide.
Recently, optical investigation of blood flow at the single-cell level has become a feasible, non-invasive method that allows inspection of the vascular system without causing damage or perturbations. This year, our interdisciplinary team of physicists and biomedical scientists performed an all-optical characterization of hemodynamics in active blood vessels of living zebrafish, by combining optical flow analysis with optical tweezers.
First, we generated a mutation in the endoglin gene, modeling a vascular disease called hereditary hemorrhagic telangiectasia (HHT), also known as Osler’s disease.1 In humans, HHT causes internal bleeding due to abnormal blood vessel development. Our zebrafish model featured a similar abnormal formation of blood vessels, including a highly dilated connection between the major trunk artery and vein, due to enlargement of endothelial cells that cannot react properly to blood flow during embryonic development.
We next employed particle image velocimetry (PIV) with bright-field microscopy—a contactless, sterile method to access blood flow velocities2—to extract spatiotemporally resolved blood flow fields, using red blood cells (RBCs) as flow tracers. We observed significant increases in blood flow velocity in the mutant fish relative to healthy fish at key time points during embryonic development. Strikingly, blood flow velocities in mutant veins resembled those normally found in arteries.
As mutant capillaries show reduced RBC flow, we used optical tweezers for weak trapping of RBCs flowing past these capillaries.1,3 We were able to reroute RBCs into mutant capillaries, effectively alleviating symptoms of the HHT phenotype.
Thus, purely by using light, we showed that the mutation causes significant changes in blood vessel diameters and blood flow behavior.1 In vivo bright-field microscopy with PIV can be applied to many more scenarios and organisms, making them relevant technologies for biomedicine.
Researchers
Robert Meissner and Cornelia Denz, Institute of Applied Physics, Westfälische Wilhelms-Universität Münster,
Germany
Wade W. Sugden and Arndt F. Siekmann, Max Planck Institute for Molecular Biomedicine, Münster, Germany
References
1. W.W. Sugden et al. Nat. Cell Biol. 19, 653 (2017).
2. M. Esseling et al. Appl. Opt. 49, 31 (2010).
3. F. Hoerner et al. J. Biophotonics 10, 110 (2017).
