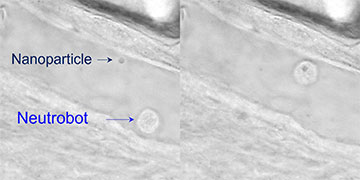
Optical tweezers guide a neutrophil microbot toward a nanoparticle (left). Next, the microbot absorbs the nanoparticle and moves it away (right). [Image: Adapted from ACS Cent. Sci., doi: 10.1021/ACSCENTSCI.2C00468] [Enlarge image]
Researchers from Jinan University, China, demonstrated optical manipulation of native neutrophils, a type of white blood cells, in live zebrafish. The so-called neutrobots were remotely guided with scanning optical tweezers to complete a variety of precise tasks without damaging surrounding tissue or triggering an immune reaction (ACS Cent. Sci., doi: 10.1021/ACSCENTSCI.2C00468).
The neutrobot technology could someday lead to medical microbots recruited from a patient’s own white blood cells that could noninvasively deliver drugs to specific locations in the body and treat inflammatory diseases without risk of rejection.
Steering cells with light
Medical microbots are promising tools for treating and preventing diseases in vivo. However, microbots currently in development are made of synthetic materials that can trigger an immune response, forcing the bots out of the body before they can complete their micro missions. Native blood cells recruited for medical microbot tasks within the body do not disrupt the host’s physiological environment, but they lack navigation precision.
In an effort to overcome these challenges, Xianchuang Zheng, Baojun Li and colleagues looked to neutrophils—cells that are already programmed to migrate through blood vessels, transport nanoparticles and remove cellular debris—and scanning optical tweezers to remotely and precisely control the cells’ movement in vivo.
In their paper, the researchers note that “optical tweezers have exhibited great potential for manipulating blood cells in vivo due to their contactless and noninvasive nature, single-cell precision, and being free from strict limitations on the specific material property.”
For the tweezers, the Jinan team employed laser beams at wavelengths of 1064 nm to minimize photothermal and photodynamic damage to biological tissue. They first demonstrated precise navigation of neutrophils in a dish using the scanning optical tweezers. Next, they took their concept one step further by optically manipulating the neutrobots in the tails of live zebrafish.
Neutrobot test drive
Before steering the neutrobots through an in vivo obstacle course, the team remotely activated the neutrophils with laser light and marked their maximum velocity at 1.3 µm per second—three times faster than neutrophils move on their own.
Next, the researchers explored three potential biomedical applications for optically manipulated neutrobots in the tail of a live zebrafish. Using the scanning optical tweezers to precisely control fluorescence-labeled neutrophils, the team demonstrated that the neutrobots could be directed through a blood vessel and into surrounding tissue (intracellular connection), navigate toward and absorb dead red blood cells via phagocytosis (elimination of cellular debris) and retrieve a plastic nanoparticle and deliver it to a set location (targeted nanomedicine delivery).
The team’s future plans for their medical microbot technology include characterizing the internal mechanisms involved with neutrophil movement and increasing the number of neutrobots simultaneously controlled in vivo to coordinate more complex medical tasks.